

BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://imtj.gmu.ac.ir/article-1-3103-en.html
2- PhD. of Exercise Physiology, BS. of Physiotherapy, Assistant Professor, Department of Physical Education and Sport Sciences, Faculty of Humanities, Saghez Branch, Islamic Azad University, Saghez, Iran. , moradi_fatah@yahoo.com
3- PhD. of Exercise Physiology, Assistant Professor, Department of Physical Education and Sport Sciences, Faculty of Humanities, Saghez Branch, Islamic Azad University, Saghez, Iran.
1. Introduction
Participating in sport activities and regular physical exercise, which are associated with structural and functional changes in the heart (of the athlete), allows for an enormous and steady increase in cardiac output or an increase in blood pressure. These changes which indicate that the exercised athlete has undergone cardiovascular reconstruction, can be seen on the Electrocardiogram (ECG) [1-3].
The main purpose of ECG interpretation in athletes is to classify ECGs into normal (no need for further evaluation) and abnormal (requires further evaluation). Natural ECGs include common training-related findings in athletes such as high QRS range related to voltage criteria for left ventricular hypertrophy, early repolarization, sinus bradycardia, sinus arrhythmia, and first-degree atrioventricular block. Abnormal results in ECG are not related to regular exercise. They could also be found in major cardiac pathological conditions. Abnormal ECG results in athletes include inversion of T wave, fall of ST segment, pathologic Q waves, long QT interval, and short QT interval [4].
Changes in the athlete’s electrocardiogram include rhythmic fluctuations (such as sinus bradycardia, sinus arrhythmia, and sinus arrest), morphological changes (including increased P wave amplitude and increased QRS voltage), and repolarization abnormalities (including ascent or descent of ST segment and decreased P wave amplitude). The most important characteristic of an athlete’s ECG is that high-intensity dynamic endurance sports are typically associated with rhythmic and conductive abnormalities in the ECG, which are due to lower endogenous heart rate and changes in sympathetic and parasympathetic tone, and the structural adaptations of the heart lead to morphological changes in the QRS complex [5].
In a review of 874 young athletes’ ECGs recorded over a 5-year period, fall of ST segment (in two or more leads), T wave flattening or inversion (in two or more leads), prolonged QT wave (greater than 0.44 seconds in men and 0.46 seconds in women) and shortening of PR interval (less than 0.12 seconds) and shortening or lengthening of corrected QT intrval (QTc) for heart rate (more than 470 milliseconds in men and more than 480 milliseconds in women and less than 340 milliseconds in both groups) were reported in the ECG of young athletes as abnormal characteristics of the ECG, which required further evaluation [6]. In another study, a comparison of resting ECGs showed lower heart rate and longer QT interval and QTc interval in professional soccer players compared to healthy volunteers of the same age who did not participate in sports competitions [7].
Toufan et al. (2012) described the most common ECG abnormalities among Iranian adolescent athletes as sinus bradycardia and incomplete right bundle branch block. Static exercise (such as weightlifting) appears to reduce left ventricular end diastolic diameter, while dynamic exercise (such as long-distance running) appears to increase left ventricular end diastolic diameter and left atrial volume index. Iranian athletes showed no differences in heart rate change parameters other than heart rate and systolic blood pressure compared to non-athletes [8]. Jorat et al. (2015) examined the effect of cardiac rehabilitation program on ECG parameters after myocardial infarction. The researchers noted improvements in the electrical activity of the heart with myocardial infarction after exercise in the rehabilitation program, and found that the cardioprotective effects of rehabilitation programs were due to improved regulation of the autonomic nervous system [9].
In a different study, Turkmen et al. (2004) to determine whether or not physiological changes (morphological and functional changes that occur as a result of regular physical exercise) lead to ventricular repolarization abnormalities in exercised athletes, they studied both exercised athletes and sedentary people of the same age and gender (as the control group). Their findings showed that heart rate, systolic blood pressure and diastolic blood pressure were similar between the two groups. The maximum QT intervals and the minimum QT intervals did not differ between the two groups of athletes and control. QT dispersion and QTc dispersion were not different between the two groups. Also, despite physiological and structural changes in the heart, no association was observed between the athlete’s heart and ventricular heterogeneity compared with the healthy sedentary control group [10].
In addition to exercise volume (intensity and duration) and type of exercise, other factors such as age, gender, and race also play a role in the development of certain ECG patterns [2]. Cal Abad (2017) showed that continuous and interval training aerobic exercise similarly increases cardiac function and autonomic modulation in mice with myocardial infarction [11], but they did not mention how exercise do affect the ECG of the subjects. Most previous studies that have examined the effect of exercise on ECG indicators are retrospective, causal-comparative (post-event) type [4-8, 10], and few experimental and prospective studies have been conducted in this area [9]. ECG is a good tool for studying the physiological adaptations of the heart to exercise [1-3]. However, training characteristics can affect ECG patterns [2]. Considering this, due to lack of findings about comparison of the effect of different aerobic training methods on ECG indicators, especially in active young girls, the present study was conducted to compare the effect of continuous and interval aerobic training on ECG of young active girls.
2. Materials and Methods
The subjects
The present study was a quasi-experimental study involving experimental (continuous aerobic training and interval aerobic training) and control groups. The measurements were in the form of pre-test (before training period) and post-test (after training period), and the young girls were physically active (with at least one year of regular exercise) and were studied in Bukan City. Availability sampling method was used in the research and the subjects were selected from among students aged 16 to 18 studying in physical education course in Fajr Girls’ Technical School of Bukan City (West Azerbaijan Province) in 2017.
The subjects were randomly assigned to the groups by replacement randomization method. First, the table of random numbers was used for simple randomization, and then the randomization program was repeated until the equilibrium of the number of subjects was obtained in three groups. Inclusion criteria of the subjects were: Non-consumption of alcohol, tobacco and any medication or sports supplements; lack of dietary nutrition; lack of any specific diseases such as cardiovascular, respiratory and musculoskeletal-orthopedic diseases in the three months before the start of the study. Exclusion criteria were: Not having regular exercises; consuming medicine, alcohol, tobacco or nutritional supplements; dietary changes; exercising other than prescribed exercises; suffering from cardiovascular, respiratory and musculoskeletal-orthopedic diseases; and failure to follow the recommendations during the study period due to injuries and stressful physical or mental-psychological events [12].
Using GPower software version 3.1.9.2 with adjustment for variance analysis test with repeated measurements (interaction effect), probability of α error=0.05, statistical power=0.90 and ŋ2=0.1, a total of 33 people was estimated as the number of subjects. However, based on the exclusion criteria, one person was excluded from the research and two others did not participate in the post-test ECG evaluation. As a result, the final samples under study were 30 people: continuous aerobic training group (n=10), interval aerobic training group (n=10) and control group (n=10). All candidates completed a health history questionnaire, a written consent form, and a physical fitness form. This research was carried out after approving by the Research Council of the Islamic Azad University, Saqqez Branch. This research was registered by the National Ethics Committee in Biomedical Research with the code IR.SSRC.REC.1398.004 and in the Iranian Clinical Trials Registration System with the Code IRCT2012070702010158N6.
2. Materials and Methods
Before beginning the training course, first, during a briefing session, the objectives, research plan and methodology, training program, laboratory evaluations (e.g. blood sampling) and the stages and schedule of the research was explained in detail to the candidates. Also, the points that the candidates should observe during the study were explained, including the cases that could led to the exclusion of the candidates from the research process, as well as the points that were required to be observed by the candidates before the pre-test and post-test evaluations.
Candidates were asked to avoid any changes in their daily diet during the research period, to practice according to the training protocol taught by the researcher, and to avoid doing physical activities in excess of the prescribed exercises. Prior to the pre-test assessments, subjects were asked to follow a few tips: to avoid doing any physical activity in excess of daily life activities 48 hours before the assessment; to keep notes of everything they eat 24 hours before the assessment, on the daily nutrition record sheet; on the pre-test day, be present for assessments after eating a regular breakfast. The assessments were performed between 8 and 10 in the morning in the presence of nursing expert of Bukan City’s Occupational Medicine Health Center, in Fajr Technical School. First, the resting ECGs of the subjects were taken. Then, the anthropometric and physiological characteristics of the candidates, including height, weight and Body Fat Percentage (BFP) were measured and their body mass indices (BMI) were calculated.
After the pre-test stage, the training course started. In both groups of continuous aerobic training and interval aerobic training, each session included warm-up (10 minutes), cooling (10 minutes), and main exercise. The main exercise included running in the sports hall. The intensity of the exercise started from 60% of the maximum heart rate and continued up to 75%. In order to observe the principle of overload, two minutes per week were added to the training time, so that duration of the main exercise increased from 20 minutes in the first week to 35 minutes at the end of the eighth week. In the first week of the main exercise of the interval training group, the ratio of interval training to active rest was 60 to 15 seconds. This ratio for each person increased per week according to their progress. But in the continuous training group, the training was done consecutively without interruption. The training protocol was conducted for eight weeks (three sessions per week) on an every-other-day basis (with no training on Fridays) and under the full supervision of the researcher [13].
After the training period, the post-test stage began. The points that the candidates had observed before the pre-test stage, again they had to observe the same points before the post-test stage. Post-test assessments were performed 48 hours after the last training session. Post-test assessments were repeated similarly to the pre-test stage and in the same order. To control the possible effects of nutrition on the ECG, the subjects were asked to write down on a daily nutrition record exactly what they ate one day prior to the pre-test assessment, and repeat the same diet on the day before the post-test assessment.
Data collection tools
Body weight was measured using a digital scale (minimum accuracy of 0.1 kg, BEURER brand, BG55 model, made in China) and height was measured using a height gauge (minimum accuracy of 0.1 cm, BALAS brand, telescopic model, made in Iran).
BMI was calculated by dividing body weight (kg) by height squared (m2). Body fat percentage was also determined using body fat analyzer (1% accuracy, CITIZEN brand, BM100 model, made in Japan).
The ECG was evaluated using a single channel 12 leads ECG device (KENZ, ECG110, Japan). To record ECG, the subjects were first asked to lie comfortably in a supine position. Any metal object such as a watch, ring, etc. was separated from the subject. Also, the subject’s clothing was manipulated in such a way that the arms, legs and chest were exposed. The device was connected to an AC power source. About 2 cm2 of ECG gel was applied for the desired areas. The device was started and the subjects’ ECGs were recorded on heat-sensitive paper with a width of 50 mm and a speed of 25 mm/s [12]. All measurements were performed on lead II and the amplitude of P, R and T waves, as well as the duration of intervals of RR, QT, PR and ST segment were recorded.
Statistical analysis
Due to the distance between the data scales, parametric tests were used for statistical analysis. Descriptive statistics (Mean±SD) were used to describe the data. The Kolmogorov-Smirnov test was used to test the normality of population distribution and the ANOVA test with repeated measurements was used to test the hypotheses. The group (continuous training/interval training/control) was considered as an intergroup factor and measurement time (pre-test/post-test) was considered as an intra-group factor. The Mauchly’s test was used to test the spherical assumption, and if the test was significant (the spherical assumption was not established), the Greenhouse-Geisser Ɛ correction factor was used. In the case of significance of interaction effects (time and group), one-way ANOVA test was used to compare the pre-test, post-test difference between the three groups, and if it was significant too, the Bonfroni post hoc test was used. The significance level was considered to be P<0.05. All statistical analyses were performed using version 22 of the Statistical Package for the Social Sciences (SPSS) software.
3. Results
The general characteristics of the subjects are presented in Table 1.
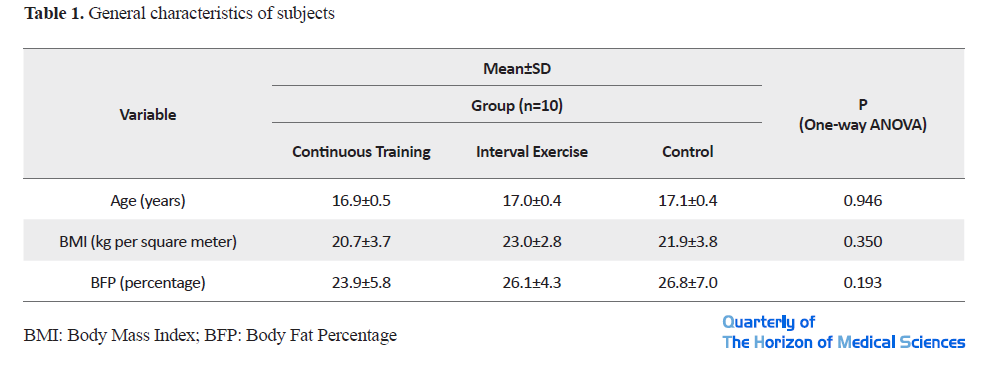
The results of the one-way ANOVA test to compare the mean age, weight, height, BMI and body fat percentage of the three groups before the study, did not show a significant difference between the groups (P<0.05). The values of ECG indicators in pre-training (pre-test) and post-training (post-test) conditions are shown in Table 2.

The results of the ANOVA test with repeated measurements for the dependent variables of the research are shown in Table 3. Based on these results, the interaction effect between time and group was significant in RR frequency, QT frequency and heart rate (P<0.05), but in other ECG indicators was not significant (P<0.05).

Due to significance of interaction effects of RR interval, QT interval, and heart rate variables, post hoc tests were used. In the case of RR interval, the one-way ANOVA test for comparing the pre-test/post-test discrepancies of the three groups was significant (F=0.027 and P=0.007).The results of the Bonfroni post hoc test showed a significant difference between pre-test, post-test discrepancies of interval training and control groups (P=0.007), but it didn’t show any significant difference between pre-test/post-test discrepancies of continuous training and control groups (P=0.079) and continuous training and interval training groups (P=0.921).
In the case of QT interval, the one-way ANOVA test for comparing the pre-test/post-test discrepancies of the three groups was significant (F=33.541 and P=0.001). The result of the Toki’s post hoc test showed a significant difference between pre-test, post-test discrepancies of continuous training and control groups (P=0.001) and interval training and control groups (P=0.027), but it didn’t show any significant difference between pre-test/post-test discrepancies of continuous training and interval training groups (P=0.173).
In the case of heart rate, the one-way ANOVA test for comparing the pre-test, post-test discrepancies of the three groups was significant (F=8.648 and P=0.001). The result of the Toki’s post hoc test showed a significant difference between pre-test, post-test discrepancies of continuous training and control groups (P=0.002) and interval training and control groups (P=0.013), but it didn’t show any significant difference between pre-test, post-test discrepancies of continuous training and interval training groups (P=0.001).
4. Discussion
The results of the present study showed that eight weeks of continuous aerobic training and interval aerobic training had no effect on the amplitude of P, R and T waves, PR interval and duration of ST segment of the active young girls, but both types of training similarly increased QT interval and decreased the heart rate. RR interval showed a significant increase only in the interval training group.
Sharma et al. (1999) evaluated ECG changes in 1000 trained elite young athletes at a high level. Their findings showed that athletes had a higher incidence of sinus bradycardia and sinus arrhythmias than non-athletes. PR interval and QRS and QT duration were longer in athletes than in non-athletes. Ascent of the ST segment was also more common in athletes than in non-athletes, and in none of the athletes with the sign of Left Ventricular Hypertrophy (LVH) did the ST segment decrease [14].
The study of Hulke and Phatak (2011) is likely to be most similar to the present study. These researchers examined the cardiac compatibility of young physical education students after twelve weeks of endurance training and showed that the P-wave amplitude, P-wave duration, PR interval, QRS wave duration, ST segment duration, ST interval, and QT interval had no significant changes, but the RR interval was significantly increased, and the heart rate was significantly decreased in male subjects. None of the above-mentioned indicators showed a significant change in the women’s group. Also, the amplitude of the T-wave in lead II did not show a significant change in any of the male and female subjects, while the highest amplitude of the T-wave showed a significant increase in men’s group (but not in women’s group). Heart rate and RR interval were inversely related. Also, exercise between tonic activity of sympathetic excitatory neurons and parasympathetic inhibitory neurons creates an imbalance in favor of greater vagal dominance. This response is mediated primarily by increased parasympathetic activity and a small decrease in sympathetic discharge. Exercise also reduces the rate of endogenous stimulation of sinoatrial pacemaker tissue. Comparing their findings with the findings of previous researchers and in interpreting ECG following exercise, the researchers noted the importance of exercise time as well as the role of frequency and intensity of exercise [12].
Mahdiabadi et al. (2013) examined the effect of eight weeks of continuous and interval aerobic training program (running in the suburbs) on heart structure and function in non-athletic men. The results of this study showed that the heart (especially the left ventricle) gets bigger after aerobic exercise. It seems that this megalocardia not only does not interfere with heart function but also improves it. Changes in the intercostal wall thickness of the heart muscle in the interval training group, and in the posterior wall thickness of the heart muscle in the continuous training group, indicate cardiac adaptation with increasing pressure due to training programs. A significant increase in the contractile performance indicators of the heart shows that continuous and interval aerobic training programs in the form of two softeners are beneficial for strengthening the heart muscle. Also, both types of training programs have similar effects on myocardial contractility [15].
Abnormal shortening or lengthening of QT interval in ECG, such as those seen in people with Mendelian forms of long or short QT syndrome, is associated with an increased risk of ventricular arrhythmias and sudden cardiac death. In addition, public studies have shown a link between smaller increases in QT interval and overall mortality, cardiovascular disease, and cardiac sudden death. In addition to genetic disorders and drug factors that can lead to a marked prolongation or shortening of QT interval, other factors are associated with less severe variability in QT interval in the general population, such as age, sex, hypertension, body mass index, low-calorie diets, electrolytes, and common genetic mutations [16]. Also, Zhang et al. (2011) found that excessive alcohol consumption was associated with longer QT intervals in men than in women. In addition, QT interval time is not associated with other modifiable factors such as consumption of coffee, tea and tobacco, and physical activity [16]. Another study reported that high physical activity was associated with increased QT interval in men rather than women. It is assumed that a higher left ventricular mass can justify this association, and that such an effect may be observed only at very high levels of physical activity [17]. Differences in population studied, levels of physical activity, and assessment of physical activity may indicate inconsistencies in study findings [16, 17]. Baronsky et al. (2013) examined the abundance of significant ECG abnormalities in 1,000 active child athletes. According to their findings, the mean RR and QTc were longer in active athletic children than in non-athletic children [18].
Conventional doctrine states that QT interval is inversely related to heart rate, so that with increasing heart rate, QT interval decreases. Akhras and Rickards (1981) examined the relationship between QT interval and heart rate during exercise and stated that QT interval is mainly determined by extrinsic factors and is not related to intrinsic heart rate [19]. Genovesi et al. (2017) examined the effects of exercise on heart rate and QT interval in healthy young people. Using a 24-hour ECG (Holter) record in healthy subjects, the researchers found that in basal heart rate, trained people had lower heart rates and higher heart rate variability than sedentary people, independent of gender differences. QTc was similar in both men and women who exercised, while there was a significant difference between women who exercised and those who did not. The researchers concluded that the cardiovascular response to exercise may be different in men and women, and that women may benefit more from increased physical activity in order to prevent cardiovascular disease and mortality. The researchers stated that the effect of exercise training on QT interval may be due to increased vagal activity on the heart at the ventricular level as a result of exercise. In addition, they did not provide a clear justification for the difference in the effect of exercise on ventricular repolarization in men and women in their study [20].
The physiological adaptations of the heart to long-term intense physical exercise causes electrocardiographic changes that are considered abnormal in untrained individuals. It is assumed that increased tone of the vagus, anatomical changes in the heart, and other lesser-known mechanisms could lead to a range of superficial ECG changes for trained athletes. It is important to pay attention to the type of physical activity, the intensity of the exercise, the athlete’s race, the body structure, and the timing of the ECG in relation to the exercise to better understand the normal range of ECG changes in athletes. Exercise improves survival after myocardial infarction.This effect may be partially justified by increased cardiac vagal activity, which reduces the risk of arrhythmias and sudden cardiac death. In fact, exercise reduces heart rate and increases heart rate variability in healthy people and in patients with myocardial infarction or heart failure. Higher heart rate, before and during exercise, and a decrease in heart rate variability in seemingly healthy individuals are associated with an increased risk of sudden cardiac death. Chronic exercise creates a resting bradycardia (at resting condition) that is thought to be partly due to increased vagal modulation. Exercise has been shown to increase RR interval, indicating the role of increased vagal tone [20].
Electrical manifestations of exercise are broadly divided into two categories: those caused by increased vagal tone and those that reflect the size of the cardiac chamber. The athlete’s normal electrocardiogram spectrum is affected by age, gender, race, and type of exercise [22]. Regular exercise leads to structural and electrical cardiac adaptations that is reflected in resting state of 12-lead ECG, so that the athlete’s ECG can be completely different from the ECG of a sedentary person of the same age, sex, and race. Common ECG changes in athletes, such as bradycardia and left ventricular hypertrophy, based on voltage criteria and early repolarization pattern, can easily be identified as normal aspects of athletic fitness and do not require further assessment, but reverse T wave after V2 lead, fall of the ST segment and Q waves, even in asymptomatic athletes, should prompt further investigation to distinguish pathology [23].
As one of the first studies in this field (as far as the knowledge of researchers in this study is concerned), the findings of the present study showed that two months of continuous and interval aerobic training to a large extent have a similar effect on resting ECG features of young active girls. Several variables appear to play a role in how exercise affect the resting ECG, which can be cited as reasons for inconsistencies in the findings of existing studies, including the characteristics of the exercises used (such as type, duration, intensity, frequency) [12, 21, 22], diet (such as electrolytes and alcohol consumption) [16], characteristics of the subjects under study (age, sex, race, level of readiness and physical activity, physical structure and genetic) [16, 17, 21, 22], health status of the subjects (hypertension, cardiovascular disease and diabetes) [16] and methods of assessment of physical activity and ECG (ECG registration time in relationship with physical activity and physical activity assessment tests) [16, 17]. Therefore, it is necessary to consider the effectiveness of these factors when interpreting the ECG in athletes and after exercise and also when comparing the findings with other studies.
Lack of dietary control, insufficient assurance of non-performance of physical activity in addition to the exercises prescribed during the study period and short duration of the training period (due to limited access to subjects) are among the limitations of the present study which paying attention to them in future research can help to complete the findings. Similar studies in other population groups (e.g. inactive, obese, or chronic obstructive pulmonary disease subjects) may be performed with a longer training period (3 or 6 months) or with different intensities that can reveal other aspects of the issue.
5. Conclusion
Based on the findings of the present study, following eight weeks of continuous and interval aerobic training, the amplitude of P, R and T waves, PR interval and duration of ST segment did not change in active young girls, but following both exercise, and similarly, QT interval increased and heart rate decreased, while only interval aerobic training increased RR interval.
Ethical Considerations
Compliance with ethical guidelines
This research has been registered by the National Ethics Committee in Biomedical Research with the Code IR.SSRC.REC.1398.004 and in the Iranian Clinical Trials Registration System with the Code IRCT2012070702010158N6.
Funding
This study was conducted with the financial support of the Vice Chancellor for Research of the Islamic Azad University, Saqqez Branch. Also, this study was extracted from the Master’s thesis of Hawzhin Azizi in Sports Physiology at Department of Physical Education and Sport Sciences, Faculty of Humanities, Saghez Branch, Islamic Azad University, (Code 24721404962001).
Authors' contributions
Final approve: All authors; Design, collecting data, writing original edition, final review: Hawzhin Azizi; Original idea, writing original edition, final review: Fatah Moradi; Interpreting data, writing first edition, final review: Saman Pashaei.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The Research Deputy of Saqqez Branch, the staff of the Occupational Medicine Health Center and the senior students of the Physical Education course of the Girls’ Technical School of Fajr in Bukan City are sincerely appreciated for their cooperation.
References
1.Corrado D, Biffi A, Basso C, Pelliccia A, Thiene G. 12-lead ECG in the Athlete: Physiological versus Pathological Abnormalities. British Journal of Sports Medicine 2009; 43:669-76. [DOI:10.1136/bjsm.2008.054759] [PMID]
2.Prakash K, Sharma S. Interpretation of the Electrocardiogram in Athletes. Canadian Journal of Cardiology. 2016; 32(4):438-51. [DOI:10.1016/j.cjca.2015.10.026] [PMID]
3.Prakash K, Sharma S. The Electrocardiogram in highly trained athletes. Clinics in Sports Medicine. 2015; 34(3):419-31. [DOI:10.1016/j.csm.2015.03.008] [PMID]
4.Drezner JA. Standardised criteria for ECG interpretation in athletes: A practical tool. British Journal of Sports Medicine. 2012; 46:i6-i8. [DOI:10.1136/bjsports-2012-091703] [PMID]
5.Fagard R. Athlete’s heart. Heart. 2003; 89(12):1455-61. [DOI:10.1136/heart.89.12.1455] [PMID] [PMCID]
6.Fuller C, Scott C, Hug-English C, Yang W, Pasternak A. Five-year experience with screening electrocardiograms in national collegiate athletic association division I athletes. Clinical Journal of Sport Medicine. 2016; 26(5):369-75. [DOI:10.1097/JSM.0000000000000318] [PMID] [PMCID]
7.Lengyel C, Orosz A, Hegyi P, Komka Z, Udvardy A, Bosnya’k, et al. Increased short-term variability of the QT interval in professional soccer players: Possible implications for arrhythmia prediction. PLoS One. 2011; 6(4):e18751. [DOI:10.1371/journal.pone.0018751] [PMID] [PMCID]
8.Toufan M, Kazemi B, Akbarzadeh F, Ataei A, Khalili M. Assessment of electrocardiography, echocardiography, and heart rate variability in dynamic and static type athletes. International Journal of General Medicine. 2012; 5:655-60. [DOI:10.2147/IJGM.S33247] [PMID] [PMCID]
9.Jorat M, Raafat S, Ansari Z, Mahdavi-Anari L, Ghanbari-Firoozabadi M. The impact of hospital-based cardiac rehabilitation on pnal average ecg parameters of the heart after myocardial infarction. Research in Cardiovascular Medicine. 2015; 4(3):e26353. [DOI:10.5812/cardiovascmed.26353v2] [PMID] [PMCID]
10.Turkmen M, Barutcu I, Esen AM, Ocak Y, Melek M, Kaya D, et al. Assessment of QT interval duration and dispersion in athlete’s heart. Journal of International Medical Research. 2004; 32(6):626-32. [DOI:10.1177/147323000403200607] [PMID]
11.Abad CCC, do Nascimento AM, dos Santos LE, Figueroa D, Ramona P, Sartori M, et al. Interval and continuous aerobic exercise training similarly increase cardiac function and autonomic modulation in infarcted mice. Journal of Exercise Rehabilitation. 2017; 13(3):257-65. [DOI:10.12965/jer.1734914.457] [PMID] [PMCID]
12.Hulke SM, Phatak MS. Cardiac adaptation to endurance training in young adult. Chronicles of Young Scientists. 2011; 2:103-8. [DOI:10.4103/2229-5186.82973]
13.Hosseini-Kakhak SAR, Rezaei Bajestani A, Shahabi Kaseb MR. Comparison of the effects of two different training methods (interval vs. continues) on aerobic fitness in 9 to 12 year-old students. Journal of Applied Exercise Physiology. 2015; 11(21):83-92. https://dc.etsu.edu/cgi/viewcontent.cgi?article=1173&context=etd
14.Sharma S, Whyte G, Elliott P, Padula M, Kaushal R, Mahon N, et al. Electrocardiographic changes in 1000 highly trained junior elite athletes. British Journal of Sports Medicine. 1999; 33(5):319-24. [DOI:10.1136/bjsm.33.5.319] [PMID] [PMCID]
15.Mahdiabadi J, Gaeini AA, Kazemi T, Mahdiabadi MA. The effect of aerobic continuous and interval training on left ventricular structure and function in male non-athletes. Biology of Sport. 2013; 30(3):207-11. [DOI:10.5604/20831862.1059302] [PMID] [PMCID]
16.Zhang Y, Post WS, Dalal D, Blasco-Colmenares E, Tomaselli GF, Guallar E. Coffee, alcohol, smoking, physical activity and qt interval duration: Results from the third national health and nutrition examination survey. PLoS One. 2011; 6(2):e17584. [DOI:10.1371/journal.pone.0017584] [PMID] [PMCID]
17.Fauchier L, Maison-Blanche P, Forhan A, D’Hour A, Lepinay P, et al. Association between heart rate-corrected QT interval and coronary risk factors in 2,894 healthy subjects (the DESIR Study). The American Journal of Cardiology. 2000; 86(5):557-9. [DOI:10.1016/S0002-9149(00)01015-8]
18.Baranowski R, Wolszakiewicz J, Biernacka EK, Kosydar M, Piotrowicz R. Frequency of Pnificant ECG abnormalities in 1000 sport active children. European Heart Journal. 2013; 34(Suppl 1):1787. [DOI:10.1093/eurheartj/eht308.1787]
19.Akhras F, Rickards AF. The relationship between QT interval and heart rate during physiological exercise and pacing. Japanese Heart Journal. 1981; 22(3):345-51. [DOI:10.1536/ihj.22.345] [PMID]
20.Genovesi S, Zaccaria D, Rossi E, Valsecchi MG, Stella A, Stramba-Badiale M. Effects of exercise training on heart rate and QT interval in healthy young individuals: are there gender differences? Europace. 2007; 9(1):55-60. [DOI:10.1093/europace/eul145] [PMID]
21.Ferst JA, Chaitman BR. The electrocardiogram and the athlete. Sports Medicine. 1984; 1(5):390-403. [DOI:10.2165/00007256-198401050-00004] [PMID]
22.Sharma S, Merghani A, Mont L. Exercise and the heart: The good, the bad, and the ugly. European Heart Journal. 2015; 36(23):1445-53. [DOI:10.1093/eurheartj/ehv090] [PMID]
23.Brosnan MJ. Athlete’s ECG - simple tips for navigation. Heart, Lung and Circulation. 2018; 27(9):1042-51. [DOI:10.1016/j.hlc.2018.04.301] [PMID]
Received: 2018/08/28 | Accepted: 2019/12/15 | Published: 2020/06/21
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





