

Volume 29, Issue 1 (Winter 2022)
Intern Med Today 2022, 29(1): 55-63 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shokoohizadeh L, Mardaneh J, Parsapour H, Khoshechin Z, Mohammadzadeh A. Prevalence of Vaginal Colonization by Group B Streptococcus and its
Associate Factors among Pregnant Women Referring to Fatemieh
Hospital in Hamadan. Intern Med Today 2022; 29 (1) :55-63
URL: http://imtj.gmu.ac.ir/article-1-3986-en.html
URL: http://imtj.gmu.ac.ir/article-1-3986-en.html
Leili Shokoohizadeh1 
 , Jalal Mardaneh2
, Jalal Mardaneh2 
 , Hamideh Parsapour3
, Hamideh Parsapour3 
 , Zahra Khoshechin4
, Zahra Khoshechin4 
 , Alireza Mohammadzadeh *
, Alireza Mohammadzadeh * 

 5
5

 , Jalal Mardaneh2
, Jalal Mardaneh2 
 , Hamideh Parsapour3
, Hamideh Parsapour3 
 , Zahra Khoshechin4
, Zahra Khoshechin4 
 , Alireza Mohammadzadeh *
, Alireza Mohammadzadeh * 

 5
5
1- Associate Professor, Department of Microbiology, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran.
2- Associate Professor, Department of Microbiology, School of Medicine, Infectious Diseases Research Center, Gonabad University of Medical Sciences, Gonabad, Iran.
3- Assistant Professor, Department of Obstetrics and Gynecology, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran.
4- Medical Student, Student Research Committee, School of Medicine, Gonabad University of Medical Sciences, Gonabad, Iran.
5- Medical Student, Student Research Committee, School of Medicine, Gonabad University of Medical Sciences, Gonabad, Iran. , alm13604@gmail.com
2- Associate Professor, Department of Microbiology, School of Medicine, Infectious Diseases Research Center, Gonabad University of Medical Sciences, Gonabad, Iran.
3- Assistant Professor, Department of Obstetrics and Gynecology, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran.
4- Medical Student, Student Research Committee, School of Medicine, Gonabad University of Medical Sciences, Gonabad, Iran.
5- Medical Student, Student Research Committee, School of Medicine, Gonabad University of Medical Sciences, Gonabad, Iran. , alm13604@gmail.com
Full-Text [PDF 659 kb]
(104 Downloads)
| Abstract (HTML) (344 Views)
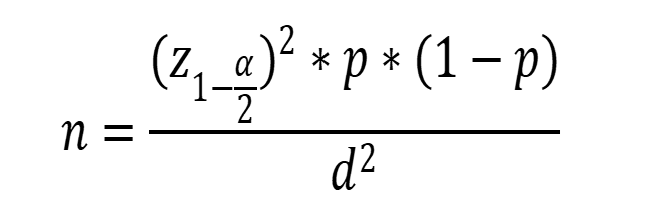
The inclusion criteria entailed willingness to participate in the research, pregnant woman between weeks 28 and 37 of gestation, and nonuse of antibiotics in the last two months. On the other hand, the exclusion criterion was pregnant women with cerclage. In this study, after obtaining written consent from the subjects, the checklist, including information on age, occupation, level of education, place of residence, gestational hypertension, parity, gravida, gestational diabetes, history of diabetes, gestational age, as well as a history of genital diseases, urinary tract infection, abortion, and preterm birth, were completed.
In order to detect GBS vaginal colonization, samples were first taken by Dacron swab, transferred to a tube containing 3 cc of Todd-Hewitt broth (THB) (with15 mg/ml of nalidixic acid and 5 mg/ml of gentamicin), and sent to the laboratory. Thereafter, the samples were placed in an incubator at 37°C and in a candle jar (to supply 5%-10% CO2 required) for 24 hours. Thereafter, the swabs were cultured on the sheep blood-agar and placed in an incubator at 37°C for 24 h. After bacterial colony growth, GBS was diagnosed based on β-hemolysis, gram staining, catalase test, bacitracin susceptibility test, sodium Hippurate-hydrolysis test, and CAMP test [17].
After collecting and entering data into SPSS software (version 16), central and dispersion indicators were used to describe quantitative variables, while frequency and frequency percentages were utilized to describe qualitative variables. In the analytical analysis, Fisher's exact test was used to investigate GBS vaginal colonization in terms of qualitative variables. Regarding the quantitative variables, the normal distribution variables were examined using the student's t-test, and the non-normal distribution variables were examined using the Mann-Whitney test after the normality of the data was determined by the Kolmogorov-Smirnov test. The significant level was considered <0.05 in all analyses.
Table 1. Individual characteristics of the studied pregnant women
Table 2. Correlation between group B streptococcus vaginal colonization and the studied variables
Table 3. Correlation of GBS virginal colonization with age, gestational age, gravida, and parity
Full-Text: (14 Views)
Introduction
Group B streptococcus (GBS) colonization of the vaginal tract affects 10% to 30% of pregnant women and is typically asymptomatic; nevertheless, it can cause endometritis, septic abortion, chorioamnionitis, urinary tract infections, and septicemia [1-3]. GBS can be carried by pregnant women in the rectum and vagina, and between 50% and 70% of them pass the bacteria on to their unborn children [4]. Pregnancy-related GBS and maternal vaginal bacterial colonization are strongly correlated with neonatal infection [5]. Neonatal infected with this bacterium develop skin or mucous membrane colonization (15–50% of newborns born to infected moms), and 1%–3% of infected newborns develop the disease [6, 7].
Early neonatal sepsis during pregnancy is related to vaginal bacterial colonization, and mother-to-child transmission happens during delivery or via the ascending channel from the mother's genital tract into the amniotic fluid [8]. Preterm birth can be related to some socio-demographic and pathological factors [9, 10]. More than half of the reported cases of GBS in neonates involve late-onset disease [11]. When it comes to late-onset illnesses, the mother is typically the source of contamination [12]. Deafness, blindness, mental impairment, and slowed growth in neonates are examples of late acute infections [13]. The rate of preterm birth in developed countries has increased from 9.7% in 1990 to 10.8% in 2004 [14, 15].
In Iran, the rate of preterm delivery is about 13.9%, and the rate of vaginal GBS colonization in mothers is estimated at 15%-18% [4, 16, 17]. Nonetheless, there are different statistics on the frequency of vaginal GBS colonization in Iran and abroad. The Centers for Disease Control and Prevention (CDC) guidelines were amended to make bacteriological screening necessary for all pregnant women 35 to 37 weeks of gestation [18]. Genital bacterial colonization in pregnant women occurs transiently, intermittently, or permanently; nonetheless, pregnant women's vaginal colonization with GBS is typically consistent throughout time. Vaginal bacterial colonization in pregnant women can be influenced by a number of factors, including ethnicity, race, maternal age, parity, marital status, socioeconomic status, education level, geographic region, occupation, smoking, presence of STDs, urinary tract infection, sexual behavior, and high body mass index [19-21]. Therefore, detection of GBS genital colonization in pregnant women is essential in order to prevent neonatal sepsis. The prevalence of GBS varies widely across geographic locations and ethnic groups.
Due to the prevalence of GBS contamination in different parts of the world, as well as the infections caused by this bacterium in pregnant women and their neonates, it is essential to ascertain the frequency of this bacterium infection in various locations. In light of the aforementioned issues, this study aimed to assess the prevalence of GBS vaginal colonization and its associated factors in pregnant patients referring to Fatemieh-Hospital in Hamadan in 2021.
Early neonatal sepsis during pregnancy is related to vaginal bacterial colonization, and mother-to-child transmission happens during delivery or via the ascending channel from the mother's genital tract into the amniotic fluid [8]. Preterm birth can be related to some socio-demographic and pathological factors [9, 10]. More than half of the reported cases of GBS in neonates involve late-onset disease [11]. When it comes to late-onset illnesses, the mother is typically the source of contamination [12]. Deafness, blindness, mental impairment, and slowed growth in neonates are examples of late acute infections [13]. The rate of preterm birth in developed countries has increased from 9.7% in 1990 to 10.8% in 2004 [14, 15].
In Iran, the rate of preterm delivery is about 13.9%, and the rate of vaginal GBS colonization in mothers is estimated at 15%-18% [4, 16, 17]. Nonetheless, there are different statistics on the frequency of vaginal GBS colonization in Iran and abroad. The Centers for Disease Control and Prevention (CDC) guidelines were amended to make bacteriological screening necessary for all pregnant women 35 to 37 weeks of gestation [18]. Genital bacterial colonization in pregnant women occurs transiently, intermittently, or permanently; nonetheless, pregnant women's vaginal colonization with GBS is typically consistent throughout time. Vaginal bacterial colonization in pregnant women can be influenced by a number of factors, including ethnicity, race, maternal age, parity, marital status, socioeconomic status, education level, geographic region, occupation, smoking, presence of STDs, urinary tract infection, sexual behavior, and high body mass index [19-21]. Therefore, detection of GBS genital colonization in pregnant women is essential in order to prevent neonatal sepsis. The prevalence of GBS varies widely across geographic locations and ethnic groups.
Due to the prevalence of GBS contamination in different parts of the world, as well as the infections caused by this bacterium in pregnant women and their neonates, it is essential to ascertain the frequency of this bacterium infection in various locations. In light of the aforementioned issues, this study aimed to assess the prevalence of GBS vaginal colonization and its associated factors in pregnant patients referring to Fatemieh-Hospital in Hamadan in 2021.
Materials and Methods
In this descriptive-analytical study, 130 pregnant women who were referred to Fatemiyeh-Hospital in Hamedan in 2021 and had a gestational age between 28 and 37 weeks had their vaginal colonization for GBS checked. Based on the study by Yasini et al. [22] and considering the significance level of 95% and the margin error of 0.05, the required sample size was calculated at 130 cases using the following formula.
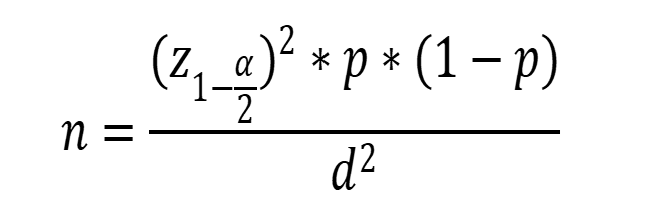
The inclusion criteria entailed willingness to participate in the research, pregnant woman between weeks 28 and 37 of gestation, and nonuse of antibiotics in the last two months. On the other hand, the exclusion criterion was pregnant women with cerclage. In this study, after obtaining written consent from the subjects, the checklist, including information on age, occupation, level of education, place of residence, gestational hypertension, parity, gravida, gestational diabetes, history of diabetes, gestational age, as well as a history of genital diseases, urinary tract infection, abortion, and preterm birth, were completed.
In order to detect GBS vaginal colonization, samples were first taken by Dacron swab, transferred to a tube containing 3 cc of Todd-Hewitt broth (THB) (with15 mg/ml of nalidixic acid and 5 mg/ml of gentamicin), and sent to the laboratory. Thereafter, the samples were placed in an incubator at 37°C and in a candle jar (to supply 5%-10% CO2 required) for 24 hours. Thereafter, the swabs were cultured on the sheep blood-agar and placed in an incubator at 37°C for 24 h. After bacterial colony growth, GBS was diagnosed based on β-hemolysis, gram staining, catalase test, bacitracin susceptibility test, sodium Hippurate-hydrolysis test, and CAMP test [17].
After collecting and entering data into SPSS software (version 16), central and dispersion indicators were used to describe quantitative variables, while frequency and frequency percentages were utilized to describe qualitative variables. In the analytical analysis, Fisher's exact test was used to investigate GBS vaginal colonization in terms of qualitative variables. Regarding the quantitative variables, the normal distribution variables were examined using the student's t-test, and the non-normal distribution variables were examined using the Mann-Whitney test after the normality of the data was determined by the Kolmogorov-Smirnov test. The significant level was considered <0.05 in all analyses.
Results
In this study, the women who participated in the study had an average age of 29.85±6.86 years. (range of 16-43). The majority of patients were between the ages of 25 and 29. In terms of employment status, most pregnant women were housewives (95.4%). Regarding education, they had junior high school (34.6%) and senior high school education (26.2%). Regarding place of residence, the majority (93.8%) lived in cities. About 70% of women had at least one prior pregnancy, and 60% of cases had at least one child. Moreover, 30% and 2.6% of cases had a history of abortion and preterm delivery, respectively. Their current mean gestational age was 33.18±3.18 weeks.
The prevalence rates of gestational hypertension, gestational diabetes, non-gestational diabetes, and genital disease in the studied pregnant women were reported as 6.9%, 14.6%, 2.3%, and 3.1%, respectively. Out of 130 pregnant women examined, 62 (47.7%) cases had a history of urinary tract infection (UTI), and prevalence of GBS vaginal colonization was 3.8% (Table 1).
The prevalence rates of gestational hypertension, gestational diabetes, non-gestational diabetes, and genital disease in the studied pregnant women were reported as 6.9%, 14.6%, 2.3%, and 3.1%, respectively. Out of 130 pregnant women examined, 62 (47.7%) cases had a history of urinary tract infection (UTI), and prevalence of GBS vaginal colonization was 3.8% (Table 1).
Table 1. Individual characteristics of the studied pregnant women
| Variable | n | % |
| Employment status | ||
| Housewife Employed |
124 6 |
95.4 4.6 |
| Education | ||
| Illiterate Elementary school Junior high school Senior high school Academic education |
3 15 45 34 33 |
2.3 11.5 34.6 26.2 25.4 |
| Place of residence | 93.8 | |
| City Village |
122 8 |
6.2 |
| Gravida | ||
| 0 1 2 3 ≥4 |
41 39 24 14 12 |
31.5 |
| Parity | ||
| 0 1 2 3 |
52 46 20 12 |
40 35.4 15.4 9.2 |
| History of abortion | ||
| Yes No |
39 91 |
30 70 |
| History of preterm birth | 6.2 | |
| Yes No |
8 122 |
93.8 |
| Gestational hypertension | 6.9 | |
| Yes No |
9 121 |
93.1 |
| Gestational diabetes | ||
| Yes No |
19 111 |
14.56 85.4 |
| Non-gestational diabetes | ||
| Yes No |
3 127 |
2.3 97.7 |
| History of genital disease | ||
| Yes No |
4 126 |
3.1 96.9 |
Based on Fisher's exact test, GBS vaginal colonization in pregnant women showed no significant relationship with occupation, education, place of residence, history of gestational hypertension, gestational diabetes, non-gestational diabetes, history of genital disease, history of abortion, history of preterm birth, and history of UTI (Table 2). The Kolmogorov-Smirnov test was utilized to ascertain the normalcy of the data with respect to quantitative variables. The results of the non-parametric Mann-Whitney test and the student's t-test indicated that there was no significant correlation between GBS vaginal colonization and age, gestational age, parity, or gravida (Table 3).
Table 2. Correlation between group B streptococcus vaginal colonization and the studied variables
| Variable | Group B streptococcus vaginal colonization | P-value | ||
| negative n (%) |
Positive n (%) |
Total | ||
| Employment status | ||||
| Housewife Employed |
120 (96.8) 5 (83.3) |
4 (3.2) 1 (16.7) |
124 6 |
0.213 |
| Education Illiterate Elementary Junior high school Senior high School Academic education |
3 (100) 15 (100) 43 (95.6) 43 (97.1) 1 (93.9) |
0 (0) 0 (0) 2 (4.4) 1 (2.9) 2 (6.1) |
3 15 45 34 33 |
0.934 |
| Place of residence | ||||
| City Village |
117 (95.9) 8 (100) |
5 (4.1) 0 (0) |
122 8 |
1.00 |
| Gestational hypertension | ||||
| Yes No |
117 (96.7) 8 (88.9) |
4 (3.3) 1 (11.1) |
121 9 |
0.306 |
| Gestational diabetes | ||||
| No Yes |
107 (96.4) 18 (94.7) |
4 (3.6) 1 (5.3) |
111 19 |
0.552 |
| Non-gestational diabetes | ||||
| No Yes |
123 (96.9) 2(66.7) |
4 (3.1) 1 (33.3) |
127 3 |
0.552 |
| History of genital disease | ||||
| No Yes |
122 (96.6) 3 (75.0) |
4 (3.2) 1 (25.0) |
126 4 |
0.147 |
| History of abortion | ||||
| No Yes |
86 (94.5) 39 (100) |
5 (5.5) 0 (0) |
91 39 |
0.321 |
| History of preterm birth | ||||
| No Yes |
117 (95.9) 8 (100) |
5 (4.1) 0 (0) |
122 8 |
1.00 |
| History of urinary tract infection | ||||
| No Yes |
67 (98.5) 58 (93.5) |
1 (1.5) 4 (6.5) |
68 62 |
0.192 |
Table 3. Correlation of GBS virginal colonization with age, gestational age, gravida, and parity
| Variable | Group B streptococcus vaginal colonization | P-value | |
| Negative Mean ± standard deviation |
Positive Mean ± standard deviation |
||
| Age (years) | 30.60±2.90 | 25.40±6.00 | 0.158 |
| Gestational age (weeks) | 33.30±18.25 | 33.10±4.03 | 0.323 |
| Gravida | 2.36±1.29 | 1.80±0.83 | 0.224 |
| Parity | 1.92±0.95 | 1.60±0.89 | 0.120 |
Discussion
All authors contributed to this article.
The aim of the current research was to determine how common GBS vaginal colonization is in expectant mothers. Accordingly, 130 expectant patients who were sent to Hamedan's Fatemieh Hospital in 2021 were chosen and given a check-up for standard prenatal treatment. In the present study, out of 130 pregnant women, 5 (3.8%) cases had GBS vaginal colonization. GBS colonization of the vagina in pregnant women showed no significant relationship with occupation, education, place of residence, history of gestational hypertension, gestational diabetes, non-gestational diabetes, history of genital disease, history of abortion, history of preterm birth, history of urinary tract infection, parity, and gravida.
In the research conducted by Rostami et al. in Isfahan in 2021, Among 200 pregnant women, 13.5% had vaginal colonization caused by GBS. Moreover, GBS vaginal colonization demonstrated no significant relationship with gravida, history of abortion, history of UTI during pregnancy, history of preterm delivery, and education. Our study's sample size and vaginal bacterial colonization prevalence were smaller than those found in Rostami et al.'s study. Consistent with the findings of the aforementioned study, our investigation revealed no noteworthy correlation between GBS vaginal colonization and gravida, abortion history, history of urinary tract infection during pregnancy, or history of premature birth [23].
In the study conducted by Kabiri et al. (2015) on 403 pregnant women in the 35-37 week of gestation in Jahrom, the prevalence rates of GBS vaginal, rectal, and rectovaginal colonization were 16.4%, 5.2%, and 7%, respectively. The positive results of the culture tests showed no statistically significant correlation with the gestational hypertension, mother age, or history of genital illnesses. However, a history of abortion, diabetes, premature birth, UTI, place of residency, nationality, educational attainment, and cesarean section significantly corrected the positive results of the culture tests [24].
In addition, the prevalence of GBS in 371 pregnant women at 35 to 37 weeks of gestation in a university hospital in Korea using routine culture methods was 4.35% [25]. The current study's findings regarding the prevalence of GBS vaginal colonization in pregnant women were less than those of Kabiri's study and the research done in Korea. In accordance with the findings of the study by Kabiri et al., in our study, there was no statistically significant correlation found between the positive results of culture tests and the following: location of residence, country, education level, history of abortion, diabetes, premature birth, urinary infection, and cesarean section.
However, the results of our investigation on the correlation between positive culture tests and maternal age, genital illness history, and gestational hypertension do not align with the findings published by Kabiri et al. This discrepancy can be ascribed to different sample sizes in the two studies. In research projects carried out by Yasini et al. [22], Jahed Bazargan et al. [26], Nazer et al. [27], and Shahbazian et al. [28], the prevalence rates of GBS vaginal colonization were reported as 9.4%, 5.3%, 14%, and 13.2%, respectively. In the study by Yasini et al., a significant relationship was seen between GBS vaginal colonization and gravida.
In the study by Jahed Bozorgan et al., positive and negative culture tests showed no significant relationship with age, nationality, education level, gravida, amniotic sac status, or mother's body temperature upon admission. In the study by Nazer et al., positive culture tests displayed no significant relationship with maternal age, gestational age, as well as a history of abortion, diabetes, and gestational hypertension. However, there was a strong correlation between gravida and vaginal bacterial colonization [27].
The prevalence of GBS vaginal colonization in Shahbazian et al.'s study was not significantly correlated with the manner of previous delivery, history of chorioamnionitis in the previous pregnancy, history of premature rupture of membranes (PROM), history of preterm delivery, age of patients, or gravida [28]. As illustrated, In the four mentioned investigations, the prevalence of GBS vaginal colonization ranged from 3.5% to 14%. Our results more closely match those of the Jahed Bozorgan et al.'s study (3.5%).
Apart from the two studies that reported a significant relationship between gravida and GBS vaginal colonization, among other demographic variables, pregnancy and delivery records did not show any association with GBS vaginal colonization, which is in line with the results of this study. In our study, less than 30% of women had a history of two or more previous pregnancies, and about 30% had no previous pregnancy history. This discrepancy can be attributed to the low prevalence of GBS vaginal colonization.
In two studies conducted by Sharifi Yazdi et al. on 250 women who were 35–37 weeks pregnant [29] and Bakhtiari et al. on 125 pregnant women at 35 to 37 weeks of gestation [30], the rates of GBS vaginal colonization using the culture and polymerase chain reaction (PCR) methods were 9.6% and 9.3%, respectively. In our study, GBS vaginal colonization was investigated by culture method. According to the higher sensitivity of PCR in the diagnosis of GBS vaginal colonization compared to the culture method [29, 30], the same problem may be partially responsible for the discrepancy in the prevalence of GBS vaginal colonization found in our study compared to that of investigations by Sharifi Yazdi et al. and Bakhtiari et al.
In a 2007 study in Rasht, 15% of 100 pregnant women at 28–37 weeks had GBS vaginal colonization, according to Amir Mozafari et al.'s findings. [17]. In their study, Zamanzad et al. examined the vaginal mucus samples of 624 mothers who were referred to Hajar Hospital in Shahrekord for term or preterm delivery or due to PROM and reported that 110 (18%) mothers were carriers of GBS. Regarding where each of the mothers stated lived, there was no discernible difference. (urban or rural) [31]. The prevalence rate of GBS vaginal colonization obtained in both studies by Zamanzad et al. and Amir Mozafari et al. is higher than the findings of our study. This disagreement in the results can be ascribed to differences in the time of the studies, the improvement of women's health status during 15-20 years, or a difference in the geographical location of the studies, which needs further investigation.
In a study conducted in an Indian hospital at 36–37 weeks gestation, 310 pregnant women had their vaginal colonization for GBS evaluated, the prevalence of colonization was 12.9%. Among the investigated factors, a significant relationship was only found between PROM and bacterial colonization, while similar to our study, no significant relationship was observed between the other investigated factors and colonization [32]. In a study by Girma et al. in 2020 in Ethiopia, the prevalence rate of GBS rectovaginal colonization in 135 pregnant women at 35 to 37 weeks of gestation was reported as 16.3%. In the same context, in a study by Iyamba et al. in Guinea, 23.07% of 104 pregnant women at a gestational age of 35–37 weeks had GBS rectovaginal colonization. [33, 34].
In the stated study, GBS vaginal colonization had a significant relationship with the history of PROM and urinary tract infection during pregnancy. Nonetheless, there was no significant relationship with age, education, place of residence, history of contraceptive use, and pregnancy. The prevalence of GBS vaginal colonization in the this study was lower than the findings of the researches by Girma et al. and Iyamba et al. in Ethiopia and Guinea. This disparity can be ascribed to differences in geographical regions, different prevalence of GBS vaginal colonization in various geographical regions, and health status of women in different areas.
Moreover, in an investigation carried out by Tesfaye et al. in Ethiopia, 182 pregnant women were studied for antibiotic resistance and variables related to GBS vaginal colonization. In the mentioned study, the prevalence of vaginal colonization was 15.9%, and among the investigated factors, only a history of preterm birth and PROM were significantly correlated with GBS vaginal colonization [35]. In our study, the history of PROM was not examined. Our findings also agree with the results of the study by Girma et al. in terms of the lack of correlation of GBS vaginal colonization with age, education, place of residence, and pregnancy.
The low prevalence of GBS vaginal colonization can be attributed to the lack of significant correlation found between the history of UTI and GBS vaginal colonization in our study, despite the higher prevalence of GBS vaginal colonization in women with a history of UTI compared to women without such a history (6.5% vs. 1.5%). Ashary et al. conducted a review study in India on the prevalence of GBS vaginal colonization in mothers of premature neonates with culture and immunological methods. When using culture techniques, the prevalence of GBS was 7.4%, but when using immunological techniques, it was 11.6%. Moreover, GBS vaginal colonization increased the risk of preterm delivery by 9.7 times [36].
Our study's prevalence of GBS vaginal colonization was lower than Ashary et al.'s findings utilizing both immunological and cultural approaches. This disparity in the results may be due to differences in the sample size (130 vs. 9,778 cases) or geographical location of the two studies. Contrary to the results of the study by Ashary et al., in the present research, no significant relationship was observed between preterm delivery and GBS vaginal colonization. This inconsistency in the results can be ascribed to the fact that the current research investigated the current GBS vaginal colonization of women with a history of preterm delivery in their previous deliveries. Nonetheless, the study by Ashary et al. assessed the relationship between GBS vaginal colonization in the current pregnancy and delivery of the same pregnancy.
Examining different risk variables for GBS vaginal colonization was one of the study's highlights. On the other hand, among the notable limitations of this research, we can refer to the fact that research subjects were only selected from the patients referring to one healthcare center; accordingly, we cannot generalize the results of the study to pregnant women in the whole city of Hamadan. It is suggested that future studies on GBS colonization in pregnant women and its effect on pregnancy outcomes employ a larger sample size and cover all pregnant women in Hamadan.
In the research conducted by Rostami et al. in Isfahan in 2021, Among 200 pregnant women, 13.5% had vaginal colonization caused by GBS. Moreover, GBS vaginal colonization demonstrated no significant relationship with gravida, history of abortion, history of UTI during pregnancy, history of preterm delivery, and education. Our study's sample size and vaginal bacterial colonization prevalence were smaller than those found in Rostami et al.'s study. Consistent with the findings of the aforementioned study, our investigation revealed no noteworthy correlation between GBS vaginal colonization and gravida, abortion history, history of urinary tract infection during pregnancy, or history of premature birth [23].
In the study conducted by Kabiri et al. (2015) on 403 pregnant women in the 35-37 week of gestation in Jahrom, the prevalence rates of GBS vaginal, rectal, and rectovaginal colonization were 16.4%, 5.2%, and 7%, respectively. The positive results of the culture tests showed no statistically significant correlation with the gestational hypertension, mother age, or history of genital illnesses. However, a history of abortion, diabetes, premature birth, UTI, place of residency, nationality, educational attainment, and cesarean section significantly corrected the positive results of the culture tests [24].
In addition, the prevalence of GBS in 371 pregnant women at 35 to 37 weeks of gestation in a university hospital in Korea using routine culture methods was 4.35% [25]. The current study's findings regarding the prevalence of GBS vaginal colonization in pregnant women were less than those of Kabiri's study and the research done in Korea. In accordance with the findings of the study by Kabiri et al., in our study, there was no statistically significant correlation found between the positive results of culture tests and the following: location of residence, country, education level, history of abortion, diabetes, premature birth, urinary infection, and cesarean section.
However, the results of our investigation on the correlation between positive culture tests and maternal age, genital illness history, and gestational hypertension do not align with the findings published by Kabiri et al. This discrepancy can be ascribed to different sample sizes in the two studies. In research projects carried out by Yasini et al. [22], Jahed Bazargan et al. [26], Nazer et al. [27], and Shahbazian et al. [28], the prevalence rates of GBS vaginal colonization were reported as 9.4%, 5.3%, 14%, and 13.2%, respectively. In the study by Yasini et al., a significant relationship was seen between GBS vaginal colonization and gravida.
In the study by Jahed Bozorgan et al., positive and negative culture tests showed no significant relationship with age, nationality, education level, gravida, amniotic sac status, or mother's body temperature upon admission. In the study by Nazer et al., positive culture tests displayed no significant relationship with maternal age, gestational age, as well as a history of abortion, diabetes, and gestational hypertension. However, there was a strong correlation between gravida and vaginal bacterial colonization [27].
The prevalence of GBS vaginal colonization in Shahbazian et al.'s study was not significantly correlated with the manner of previous delivery, history of chorioamnionitis in the previous pregnancy, history of premature rupture of membranes (PROM), history of preterm delivery, age of patients, or gravida [28]. As illustrated, In the four mentioned investigations, the prevalence of GBS vaginal colonization ranged from 3.5% to 14%. Our results more closely match those of the Jahed Bozorgan et al.'s study (3.5%).
Apart from the two studies that reported a significant relationship between gravida and GBS vaginal colonization, among other demographic variables, pregnancy and delivery records did not show any association with GBS vaginal colonization, which is in line with the results of this study. In our study, less than 30% of women had a history of two or more previous pregnancies, and about 30% had no previous pregnancy history. This discrepancy can be attributed to the low prevalence of GBS vaginal colonization.
In two studies conducted by Sharifi Yazdi et al. on 250 women who were 35–37 weeks pregnant [29] and Bakhtiari et al. on 125 pregnant women at 35 to 37 weeks of gestation [30], the rates of GBS vaginal colonization using the culture and polymerase chain reaction (PCR) methods were 9.6% and 9.3%, respectively. In our study, GBS vaginal colonization was investigated by culture method. According to the higher sensitivity of PCR in the diagnosis of GBS vaginal colonization compared to the culture method [29, 30], the same problem may be partially responsible for the discrepancy in the prevalence of GBS vaginal colonization found in our study compared to that of investigations by Sharifi Yazdi et al. and Bakhtiari et al.
In a 2007 study in Rasht, 15% of 100 pregnant women at 28–37 weeks had GBS vaginal colonization, according to Amir Mozafari et al.'s findings. [17]. In their study, Zamanzad et al. examined the vaginal mucus samples of 624 mothers who were referred to Hajar Hospital in Shahrekord for term or preterm delivery or due to PROM and reported that 110 (18%) mothers were carriers of GBS. Regarding where each of the mothers stated lived, there was no discernible difference. (urban or rural) [31]. The prevalence rate of GBS vaginal colonization obtained in both studies by Zamanzad et al. and Amir Mozafari et al. is higher than the findings of our study. This disagreement in the results can be ascribed to differences in the time of the studies, the improvement of women's health status during 15-20 years, or a difference in the geographical location of the studies, which needs further investigation.
In a study conducted in an Indian hospital at 36–37 weeks gestation, 310 pregnant women had their vaginal colonization for GBS evaluated, the prevalence of colonization was 12.9%. Among the investigated factors, a significant relationship was only found between PROM and bacterial colonization, while similar to our study, no significant relationship was observed between the other investigated factors and colonization [32]. In a study by Girma et al. in 2020 in Ethiopia, the prevalence rate of GBS rectovaginal colonization in 135 pregnant women at 35 to 37 weeks of gestation was reported as 16.3%. In the same context, in a study by Iyamba et al. in Guinea, 23.07% of 104 pregnant women at a gestational age of 35–37 weeks had GBS rectovaginal colonization. [33, 34].
In the stated study, GBS vaginal colonization had a significant relationship with the history of PROM and urinary tract infection during pregnancy. Nonetheless, there was no significant relationship with age, education, place of residence, history of contraceptive use, and pregnancy. The prevalence of GBS vaginal colonization in the this study was lower than the findings of the researches by Girma et al. and Iyamba et al. in Ethiopia and Guinea. This disparity can be ascribed to differences in geographical regions, different prevalence of GBS vaginal colonization in various geographical regions, and health status of women in different areas.
Moreover, in an investigation carried out by Tesfaye et al. in Ethiopia, 182 pregnant women were studied for antibiotic resistance and variables related to GBS vaginal colonization. In the mentioned study, the prevalence of vaginal colonization was 15.9%, and among the investigated factors, only a history of preterm birth and PROM were significantly correlated with GBS vaginal colonization [35]. In our study, the history of PROM was not examined. Our findings also agree with the results of the study by Girma et al. in terms of the lack of correlation of GBS vaginal colonization with age, education, place of residence, and pregnancy.
The low prevalence of GBS vaginal colonization can be attributed to the lack of significant correlation found between the history of UTI and GBS vaginal colonization in our study, despite the higher prevalence of GBS vaginal colonization in women with a history of UTI compared to women without such a history (6.5% vs. 1.5%). Ashary et al. conducted a review study in India on the prevalence of GBS vaginal colonization in mothers of premature neonates with culture and immunological methods. When using culture techniques, the prevalence of GBS was 7.4%, but when using immunological techniques, it was 11.6%. Moreover, GBS vaginal colonization increased the risk of preterm delivery by 9.7 times [36].
Our study's prevalence of GBS vaginal colonization was lower than Ashary et al.'s findings utilizing both immunological and cultural approaches. This disparity in the results may be due to differences in the sample size (130 vs. 9,778 cases) or geographical location of the two studies. Contrary to the results of the study by Ashary et al., in the present research, no significant relationship was observed between preterm delivery and GBS vaginal colonization. This inconsistency in the results can be ascribed to the fact that the current research investigated the current GBS vaginal colonization of women with a history of preterm delivery in their previous deliveries. Nonetheless, the study by Ashary et al. assessed the relationship between GBS vaginal colonization in the current pregnancy and delivery of the same pregnancy.
Examining different risk variables for GBS vaginal colonization was one of the study's highlights. On the other hand, among the notable limitations of this research, we can refer to the fact that research subjects were only selected from the patients referring to one healthcare center; accordingly, we cannot generalize the results of the study to pregnant women in the whole city of Hamadan. It is suggested that future studies on GBS colonization in pregnant women and its effect on pregnancy outcomes employ a larger sample size and cover all pregnant women in Hamadan.
Conclusion
Although the low rate of GBS vaginal colonization among pregnant patients referred to Fatemiyeh-Hospital in Hamedan, the disease's detrimental effects on pregnancy outcomes make screening for GBS infection, prompt diagnosis and treatment, and research into the risk factors contributing to the illness absolutely necessary.
Ethical Considerations
Compliance with ethical guidelines
The Gonabad University of Medical Sciences' regional committee on research ethics accepted this work under the ethics code IR.GMU.REC.1400.027.Funding
The Gonabad University of Medical Sciences' Deputy of Research and Technology provided funding for this study (grant number: A-10-2062-2).
Authors' contributionsAll authors contributed to this article.
Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgments
My most profound appreciation goes to the director and staff of the Fatemieh Educational and Treatment Center laboratory, who helped me complete this thesis and collect data.References
- Turner C, Turner P, Po L, Maner N, De Zoysa A, Afshar B, et al. Group B streptococcal carriage, serotype distribution and antibiotic susceptibilities in pregnant women at the time of delivery in a refugee population on the Thai-Myanmar border. MC Infect Dis. 2012;12:34. [DOI: 10.1186/1471-2334-12-34] [PMID] [PMCID]
- Huber CA, McOdimba F, Pflueger V, Daubenberger CA, Revathi G. Characterization of invasive and colonizing isolates of Streptococcus agalactiae in East African adults. J Clin Microbiol. 2011;49(10):3652-5. [DOI: 10.1128/JCM.01288-11] [PMID]
- Manning SD, Lewis MA, Springman AC, Lehotzky E, Whittam TS, Davies D. Genotypic diversity and serotype distribution of group B streptococcus isolated from women before and after delivery. Clin Infect Dis. 2008;46(12):1829-37. [DOI: 10.1086/588296] [PMID] [PMCID]
- Cunningham F, Leveno K, Bloom S, Spong CY, Dashe J. Williams obstetrics, 24e: Mcgraw-hill; 2014. [Link]
- Palmeiro JK, Dalla-Costa LM, Fracalanzza SEL, Botelho ACN, da Silva Nogueira K, Scheffer MC, et al. Phenotypic and
genotypic characterization of group B streptococcal isolates in southern Brazil. J Clin Microbiol. 2010;48(12):4397-403. [DOI: 10.1128/JCM.00419-10] [PMID] [PMCID] - Madzivhandila M, Adrian PV, Cutland CL, Kuwanda L, Schrag SJ, Madhi SA. Serotype distribution and invasive potential of group B streptococcus isolates causing disease in infants and colonizing maternal-newborn dyads. PLoS One. 2011;6(3):e17861. [DOI: 10.1371/journal.pone.0017861] [PMID] [PMCID]
- Convert M, Martinetti Lucchini G, Dolina M, Piffaretti JC. Comparison of LightCycler PCR and culture for detection of group B streptococci from vaginal swabs. Clin Microbiol Infect. 2005;11(12):1022-6. [DOI: 10.1111/j.1469-0691.2005.01275.x] [PMID].
- Dhanoa A, Karunakaran R, Puthucheary SD. Serotype distribution and antibiotic susceptibility of group B streptococci in pregnant women. Epidemiol Infect. 2010;138(7):979-81. [DOI: 10.1017/S0950268809991105] [PMID]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75-84. [DOI: 10.1016/S0140-6736(08)60074-4] [PMID] [PMCID]
- Reedy NJ. Born too soon: the continuing challenge of preterm labor and birth in the United States. J Midwifery Womens Health. 2007;52(3):281-90. [DOI: 10.1016/j.jmwh.2007.02.022] [PMID]
- McGee L, Schrag S, Verani JR. Prevention of perinatal Group B streptococcal disease; revised guidelines from CDC. 2010. [Link]
- Schuchat A. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev. 1998;11(3):497-513. [DOI: 10.1128/CMR.11.3.497] [PMID] [PMCID]
- Raabe VN, Shane AL. Group B streptococcus (Streptococcus agalactiae). Microbiology spectrum. 2019;7(2):10-128. [DOI: 10.1128/microbiolspec.GPP3-0007-2018] [PMID] [PMCID]
- Nordenvall M, Sandstedt B. Chorioamnionitis in relation to gestational outcome in a Swedish population. Eur J Obstet Gynecol Reprod Biol. 1990;36(1-2):59-67. [DOI: 10.1016/0028-2243(90)90050-b] [PMID]
- Tucker J, McGuire W. Epidemiology of preterm birth. BMJ. 2004;329(7467):675-8. [DOI: 10.1136/bmj.329.7467.675] [PMID] [PMCID]
- Khataie G, Shahrokhi N. Bacteriologic and serologic diagnosis of group B streptococci in pregnant women, neonates and infants. Tehran Univ Med J. 1998;56(6):54-60. [Link]
- Amirmozafari N, Mansour Ghanaei M, Sadr Nouri B, Farhadi Tooli L. Survey Prevalence of Group B Streptococci in Genital Tract Women in 28-37 Weeks Pregnancy. Journal of Guilan University of Medical Sciences. 2006;15(59):91-6. [Link]
- Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. MMWR Recomm Rep. 2002;51(RR-11):1-22. [PMID]
- Morgan JA, Zafar N, Cooper DB. Group B Streptococcus and pregnancy. Treasure Island (FL): StatPearls Publishing. 2023. [PMID]
- Goel N, Wattal C, Gujral K, Dhaduk N, Mansukhani C, Garg P. Group B Streptococcus in Indian pregnant women: Its prevalence and risk factors. Indian J Med Microbiol. 2020;38(3 & 4):357-361. [DOI: 10.4103/ijmm.IJMM_20_333] [PMID]
- Jisuvei SC, Osoti A, Njeri MA. Prevalence, antimicrobial susceptibility patterns, serotypes and risk factors for group B streptococcus rectovaginal isolates among pregnant women at Kenyatta National Hospital, Kenya; a cross-sectional study. BMC Infect Dis. 2020;20(1):302. [DOI: 10.1186/s12879-020-05035-1] [PMID] [PMCID]
- Yasini M, Moniri R, Ghorbaali Z, Ansaripour L, Movahedinejad M, Yadegarsalehi M. Prevalence rate, Antibiotic susceptibility and Colonization risk factors of Group B Streptococcus in genital tract of pregnant women. medical journal of mashhad university of medical sciences. 2014;57(5):676-83. [DOI: 10.22038/mjms.2014.3406]
- Rostami S, Moeineddini L, Ghandehari F, Khorasani MR, Shoaei P, Ebrahimi N. Macrolide-resistance, capsular genotyping and associated factors of group B Streptococci colonized pregnant women in Isfahan, Iran. Iran J Microbiol. 2021;13(2):183-89. [DOI: 10.18502/ijm.v13i2.5979] [PMID]
- Kabiri S, Kargar-Jahromi Z, Solhjoo K, Sadeghi-Jahromi S. Frequency of Group B Streptococcus Colonization in pregnant women in Jahrom, 2014. Journal of Jahrom University of Medical Sciences. 2016;14(1):19-26. [DOI: 10.29252/jmj.14.1.19]
- Song KE, Hwang N, Ham JY, Cha HH, Chong GO, Lee NY. Prevalence of Group B Streptococcus Colonization in Pregnant Women at a University Hospital in Korea. Clin Lab. 2022;68(8). [DOI: 10.7754/Clin.Lab.2021.211126] [PMID]
- Jahed T, Shariati MK, Darabi P, Karimi A. Frequency of group B streptococcus colonization and antibiogram in women at 35-37 weeks of gestation visited in prenatal clinic of Mahdieh Hospital in 2008. Pajoohandeh Journal. 2011;16(3):139-43. [Link]
- Nazer MR, Rafiei Alavi E, Nazer E, Khamechi M. Prevalence of Group B Streptococcus Vaginal Colonization in The Third Trimester of Pregnancy. SSU_Journals. 2011;19(1):13-23. [DOI: 10.18502/ijrm.v16i12.3679]
- Shahbazian N, Rajabzadeh AR, Alavi SM. Prevalence of group-bstreptococcal colonization in vagina and rectum of 35-37 weeks pregnant women and its sensitivity to antibiotics. JUNDISHAPUR SCIENTIFIC MEDICAL JOURNAL. 2007;6(54):294-8. [Link]
- Sharifi Yazdi K, Bakhtiari R, Mobasseri G, Soltan Dallal Mohammad M, Khalili Mohammad B. Study of molecular epidemiologic of Group B Streptococcus colonization in pregnant women by PCR method. VHL Regional Portal. 2011;5(2):51-60. [Link]
- Bakhtiari R, Dallal MMS, Mehrabadi JF, Heidarzadeh S, Pourmand MR. Evaluation of culture and PCR methods for diagnosis of group B streptococcus carriage in Iranian pregnant women. Iran J Public Health. 2012;41(3):65-70. [PMID] [PMCID]
- Zamanzad B. A study determine the prevalence of vaginal group B streptococcal carriers in pergnant women. Journal of Shahid Sadoughi University of Medical Sciences and Health Services. 2002;10(3):27-31. [Link]
- Warrier LM, Joy S, Bashir RA. Group B Streptococcal colonization among pregnant women and neonates in a tertiary care hospital in South India. Indian J Pediatr. 2022;89(12):1187-94. [DOI: 10.1007/s12098-022-04120-4] [PMID] [PMCID]
- Girma W, Yimer N, Kassa T, Yesuf E. Group B Streptococcus Recto-Vaginal Colonization in Near-Term Pregnant Women, Southwest Ethiopia. Ethiop J Health Sci. 2020;30(5):687-96. [DOI: 10.4314/ejhs.v30i5.7] [PMID]
- Iyamba J-ML, Mongane PM, Lukukula CM, Ngbandani BK, Tshimpangila JD, Vihembo GgM, et al. Vaginal Colonization and Antibiotic Susceptibility Pattern of Group B Streptococcus Isolated from Pregnant Women in Maternité de l’Hôpital Des
Soeurs de Pauvres de Bergame de Kimbanseke, Kinshasa, Democratic Republic of Congo. Advances in Microbiology. 2021;11(7):335-41. [DOI: 10.4236/aim.2021.117026] - Tesfaye A, Melese A, Derbie A. Antimicrobial resistance profile and associated factors of group B Streptococci colonization among pregnant women attending antenatal clinics in Jigjiga, Southeast Ethiopia. Int J Microbiol. 2022;2022:9910842. [DOI: 10.1155/2022/9910842] [PMID] [PMCID]
- Ashary N, Singh A, Chhabria K, Modi D. Meta-analysis on prevalence of vaginal group B streptococcus colonization and preterm births in India. J Matern Fetal Neonatal Med. 2022;35(15):2923-2931. [DOI: 10.1080/14767058.2020.1813705] [PMID]
Type of Study: Original |
Subject:
Basic Medical Science
Received: 2023/06/3 | Accepted: 2023/06/12 | Published: 2023/06/19
Received: 2023/06/3 | Accepted: 2023/06/12 | Published: 2023/06/19
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

