

Volume 28, Issue 1 (Winter 2021)
Intern Med Today 2021, 28(1): 128-139 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Delavarian Z, Hashemi S I, Vazifeh Mostaan L, Mozaffari Jovin S, Biabani A, Ghazi A. Comparing the Salivary Zinc Finger Protein 510 Concentration in Patients With Oral Squamous Cell Carcinoma and Healthy Individuals. Intern Med Today 2021; 28 (1) :128-139
URL: http://imtj.gmu.ac.ir/article-1-3600-en.html
URL: http://imtj.gmu.ac.ir/article-1-3600-en.html
Zahra Delavarian1 

 , Seyyed Issac Hashemi2
, Seyyed Issac Hashemi2 

 , Leyla Vazifeh Mostaan3
, Leyla Vazifeh Mostaan3 

 , Sina Mozaffari Jovin4
, Sina Mozaffari Jovin4 
 , Alireza Biabani5
, Alireza Biabani5 

 , Ala Ghazi *6
, Ala Ghazi *6 




 , Seyyed Issac Hashemi2
, Seyyed Issac Hashemi2 

 , Leyla Vazifeh Mostaan3
, Leyla Vazifeh Mostaan3 

 , Sina Mozaffari Jovin4
, Sina Mozaffari Jovin4 
 , Alireza Biabani5
, Alireza Biabani5 

 , Ala Ghazi *6
, Ala Ghazi *6 


1- Oral and Maxillofacial Diseases Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Department of Clinical Biochemistry, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
3- Department of ORL-Head & Neck Surgery, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Department of Medical Genetics, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
5- Dentist, Mashhad, Iran.
6- Oral and Maxillofacial Diseases Research Center, Mashhad University of Medical Sciences, Mashhad, Iran. ,ghazial@mums.ac.ir
2- Department of Clinical Biochemistry, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
3- Department of ORL-Head & Neck Surgery, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Department of Medical Genetics, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
5- Dentist, Mashhad, Iran.
6- Oral and Maxillofacial Diseases Research Center, Mashhad University of Medical Sciences, Mashhad, Iran. ,
Full-Text [PDF 4099 kb]
(667 Downloads)
| Abstract (HTML) (2900 Views)
Full-Text: (832 Views)
Introduction
One of the most important cancers (the sixth prevalent cancer) in the world is oral cancer. In 2018, about 300,000 new cases of oral cancer and a mortality number of 150,000 worldwide were reported [1, 2]. The prevalence of this cancer varies around the world geographically up to 20 times in different areas of the world [2, 3]. The prevalence of oral cancer is significantly higher in Asia compared to developed countries [2, 4]. The oral cancer prevalence in Iran and other countries in South Asia such as India, Pakistan and Bangladesh is 20-36.3 per 100,000 people [2].
Oral Squamous Cell Carcinoma (OSCC) is the most common malignancy of oral cavity and accounts for at least 90% of all oral malignancies [4, 5]. Currently, the OSCC is diagnosed based on oral examination and histopathological findings [1, 6]. Biopsy of suspected malignancies is the standard method to determine their nature [6, 7]. In addition to the ailment caused by invasive biopsy, it is possible that precancerous non-homogenous lesions prevent determining the site of the biopsy which is significant in the histopathological assessment of oral cancer. Grading is the histopathological assessment of lesions’ degree of similarity to normal squamous epithelium and a method for evaluating the amount of creatine production. Lesions are graded on a three-point (grade I, II, III) or four-point scales. Poorly differentiated lesions have higher grades [8]. Although there are significant advances in treatment of oral cancer including surgery, radiation therapy, chemotherapy, or their combination, but about 50% of patients suffering from oral cancer survive for 5 years [9, 10]. There is still no effective method to accurately and easily screen oral cancer.
Diagnosis of novel biomarkers is essential for the detection of early malignant oral lesions, especially in those at higher risk [11]. Saliva is a good candidate for detecting biomarkers of oral cancer. Saliva is produced by three main salivary glands including parotid glands, submandibular glands, and sublingual glands, and to a lesser extent by the sub-salivary glands in the lips, cheeks, tongue, palate, and glandular ducts [12]. Using saliva to screen oral cancer is an easier and less invasive method compared to blood sampling or biopsy. Moreover, it is more easily tolerated by patients [6].
Zinc finger protein 510 is a protein that is encoded by the ZNF510 gene in humans. ZNF510 protein is a member of a zinc-finger protein family with about 30 amino acids and zinc ions. It is involved in the control of cell growth, proliferation, differentiation, and programmed death. This protein has been observed in the saliva of patients with OSCC [9]. To our knowledge, only one study has assessed the level of ZNF510 in the saliva of patients with OSCC [9, 13]. In this regard, this study aims to compare the level of ZNF510, as a novel biomarker for diagnosis of OSCC in the early stages, in the saliva of patients with OSCC and healthy individuals.
Materials and Methods
This cross-sectional analytical study was conducted on patients with OSCC and healthy individuals referred to Omid and Imam Reza hospitals affiliated to Mashhad University of Medical Sciences in Iran in 2019. In patients with OSCC, clinical and pathological examination had confirmed the presence of disease and had other inclusion criteria; i.e. age >18 years, consent to participate in the study [9]. Exclusion criteria were any systemic autoimmune disease, existence of oral mucosal lesions other than OSCC, and pregnancy or lactation [9]. According to the results of Jou et al. [9] and at the confidence level of 95%, a test power of 80%, and a type I error level of 50%, the sample size was calculated 9 for each group. This number was increased to 21 for further confidence (21 patients with OSCC and 21 healthy individuals). Prior to study, the procedure was explained to participants, and they signed a written informed consent form to enter the study. Then, their demographic characteristics and the lesion characteristics in the patient group (including lesion type, lesion site, and the histopathology grade of lesion) were recorded. Saliva sampling was then performed. Unstimulated saliva was used for saliva sampling because stimulated saliva has a low concentration of biomarkers and makes it difficult to detect [14]. Participants were advised to avoid smoking and alcohol use for 24 hours before collecting saliva. On the day of sampling, patients were not allowed to smoke, eat, drink or brush for 1-1.5 hour before collecting samples. Spitting method was used to collect unstimulated saliva [15]. Participants were asked to collect saliva in their mouths and then put it into a sterile plastic falcon tube. This was done every 60 seconds for 5-15 minutes between 9-12 a.m. Approximately 5 mL of saliva was collected by this method. During sampling, participants were in a sitting position with slightly forward posture, feeling completely comfortable.
The samples kept in the ice flask were transferred to the central laboratory of Mashhad University of Medical Sciences and stored in a refrigerator at -20°C for up to 3 days. The samples were thawed at room temperature and were then centrifuged for 15 minutes (rpm at 3000) immediately after defrosting to separate squamous cells, debris and mucus. The supernatant solution was collected and poured into 1.5-mL microtubes and stored at -80°C in the refrigerator. In the next step, ZNF510 (part per million or PPM) values were evaluated using an ELISA Kit (ZellBio GmbH, Germany) and ELISA method. For this purpose, each sample was examined twice by an experienced technician who was unaware of allocation.
In summary, the expriment steps were as following: Preparation of samples and standardization, adding 40 µl of samples, 10 µl of ZNF510, 50 µl of standard solution, and 50 µl of Streptavidin-HRP and reacting for 60 minutes at 37°C, Washing the plate 5 times with 300 µl diluted buffer, adding 50 µl of chromogen solution A and 50 µl of solution B and incubating for 10 minutes at 37°C, adding 50 µl of stop solution, reading optical density within 10 minutes at a wavelength of 450 nm, performing calculations and presenting reports.
Collected data were analyzed in SPSS software, vertion .23. For this purpose, Mean±SD error of ZNF510 concentration were reported for saliva samples of healthy individuals and patients with OSCC. Kolmogorov-Smirnov, independent t-test, chi-square, Pearson correlation test, Kruskal-Wallis and analysis of covariance were used to analyze the data. Significance level was set at 0.05 in all statistical tests.
Results
In this study, 42 individuals participated including 29 women (69%) and 13 men (31%) with a mean age of 60.62±10.77 years ranged 42-75 years. There was no significant difference between the two groups in terms of gender (P=0.317), but the mean of age in the healthy group was significantly lower than in the patient group (P<0.001).
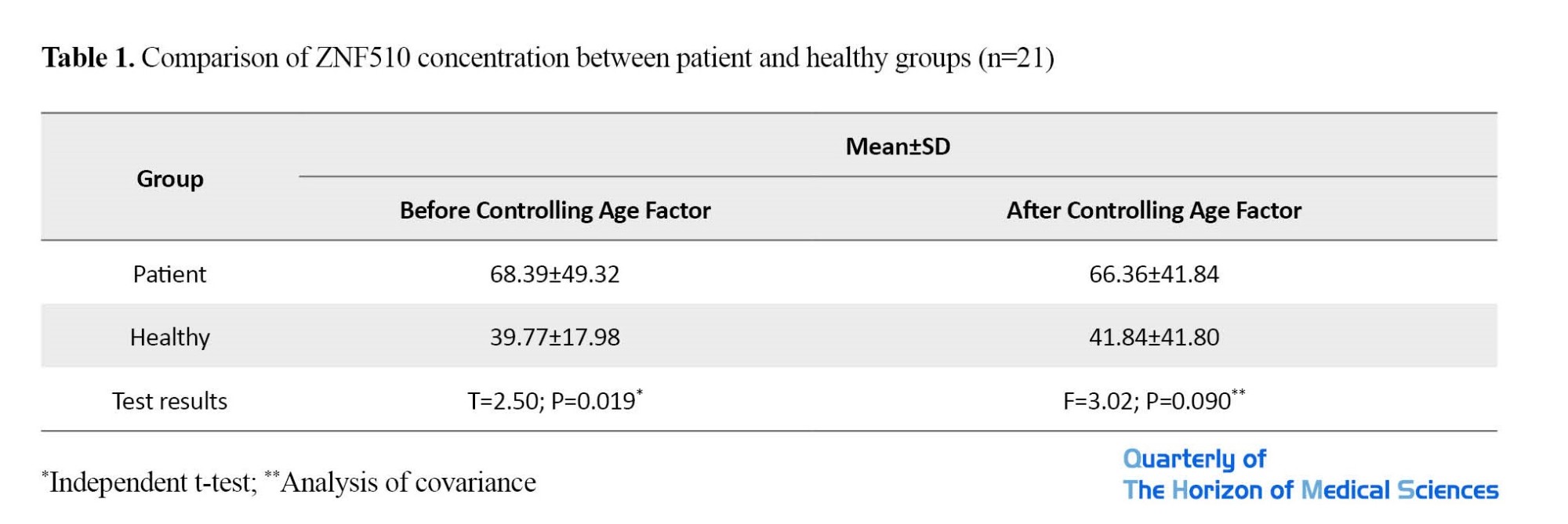
Table 1 and Figure 1, shows the mean and standard deviation of ZNF510 level. As can be seen, the mean of ZNF510 (ppm) in the patient group was significantly higher than in the healthy group according to the results of independent t-test (P=0.019); however, after considering age as a confounding variable, although the mean of ZNF510 in the patient group was higher, this difference was not significant (P=0.090).

Therefore, age affected the amount of ZNF510. As it can be seen from Figure 2, in both groups, age had a direct but weak and insignificant relationship with ZNF510. In the patient group, the mean of ZNF510 was lower in women than in men, but the difference was not significant (P=0.555). In the healthy group, the mean of ZNF510 was higher in women than in men, but the difference was not significant (P=0.606).

The clinical examination of lesions showed that the lowest and highest mean of ZNF510 were related to ulcer and prominent lesions, respectively. The mean of ZNF510 was not significantly different in terms of lesion type (P=0.522). Furthermore, in terms of lesion site, the lowest and highest mean of ZNF510 were observed on the gums and lips, respectively. The mean of ZNF510 was not significantly different in terms of of lesion site (P=0.089).
Table 2 presents the mean, standard deviation, minimum, maximum, and mean of the ZNF510 level categorized by different grades of lesion histopathology in the patient group. In patients with histopathological grade I, the lowest and highest levels of ZNF510 were reported 12.5 and 83.75 ppm; and in patients with histopathology grade II, they were 107.7 and 167.5 ppm, respectively. The mean of ZNF510 in patients with lesion grade II was significantly higher than in those with lesion grade I (P<0.001). It should be noted that 6 patients had an unknown degree of lesion histopathology. Therefore, the information in Table 2 is related to 15 patients with OSCC.

Discussion
The current study aimed to measure the salivary ZNF510 protein concentration in patients with OSCC compared to healthy individuals. In patients with OSCC, the mean of ZNF510 in those with lesion grade II was significantly higher than in those with lesion grade I. Moreover, the concentration of ZNF510 protein with the prominent exophytic tupe was higher than other clinical features, and based on the lesion site, the mean of ZNF510 in patients with lip lesions was higher than in others. However, the mean of ZNF510 was not significantly different between the two groups in terms of lesion type and site.
As mentioned before, oral cancer is the sixth most prevalent cancer worldwide, accounting for approximately 4% of all cancer cases [16]. Although the oral cavity may be examined directly, oral cancer sometimes is not diagnosed until the final stages. Biopsy and histopathological assessment are the gold standard methods for definitive diagnosis of oral cancer, including OSCC [6]. Studies have shown that saliva can be used for early diagnosis of OSCC along with biomarkers such as metallopeptidase-9 and Chemerin [12]. ZNF proteins are a group of proteins with a wide range of molecular functions involved in the regulation of several cellular processes, and their function is very different. The function of ZNF proteins seems to be related to the control of growth, proliferation, differentiation, and programmed death of cell (apoptosis) [17, 18]. Some studies have examined different types of ZNF proteins in head and neck cancers and OSCC [19-22]. One study by Jou et al. in 2011 examined the amount of ZNF510 protein in the saliva of patients with OSCC [9]. According to them, ZNF510 peptide levels were significantly increased in the saliva of patients with OSCC [9]. They indicated that ZNF510 level was significantly related to the oral cancer stages where it was higher in T3+ T4 stages compared to T1+ T2 stages. Moreover, they reported that the sensitivity and specificity in the early diagnosis and progression stage of the tumor was 96%. They concluded that the detection of ZNF510 peptide level is related to the progression of OSCC, and it is effective and useful for accurate and early diagnosis [9]. In our study, the mean of ZNF510 protein concentration in the saliva of patients with OSCC was significantly higher than in healthy individuals, but this difference was not significant between the two groups after controlling the age factor as a confounding factor, indicating that age factor affects the salivary ZNF510 concentration. Therefore, saliva protein levels are changed by increasing age, and there is an age-related effect on the secretion of a particular compound in saliva. Although the relationship between ZNF510 concentration and age in our study was weak, it can be claimed that the ZNF510 protein concentration in saliva increases by the increase of age.
Conclusion
The ZNF510 protein concentration is significantly higher in patients with OSCC compared to healthy individuals. However, after considering age as a confounding variable, this difference is not significant. It is recommended to conduct more studies by considering the effect of age factor.
It should be noted that the healthy group was selected from among the patients‘ healthy companions (first degree relatives) in order to homogenize the two groups in terms of heritage. One the other hand, the ZNF510 concentration was significantly higher in patients with lesion grade II compared to those with grade I. Therefore, ZNF510 in saliva may play a role as a possible biomarker for early diagnosis and progression of OSCC. However, more studies are needed with higher sample sizes and examinations of lesions grades III and IV in order to generalize the findings. The results of this study can be regarded as the beginning of further studies on the diagnostic methods of OSCC.
Ethical Considerations
Compliance with ethical guidelines
The study has an ethical approval obtained from the Research Ethics Committee of Mashhad University of Medical Sciences (Code: IR.MUMS.DENTISTRY.REC.1397.118).
Funding
This study was extracted from a dissertation in Dentistry approved by Mashhad Dental School. It was funded by the Deputy for Research of MasMashhad University of Medical Sciences.
Authors' contributions
Study design, data collection, writing, editing & review, and final approval: All authors; Conceptualization: Alla Ghazi.
Conflicts of interest
The authors declare no conflict of interest.
References
Acknowledgements
The authors would like to thank the Vice-Chancellor for Research of Mashhad University of Medical Sciences for the financial support, and all participants for their cooperation.
One of the most important cancers (the sixth prevalent cancer) in the world is oral cancer. In 2018, about 300,000 new cases of oral cancer and a mortality number of 150,000 worldwide were reported [1, 2]. The prevalence of this cancer varies around the world geographically up to 20 times in different areas of the world [2, 3]. The prevalence of oral cancer is significantly higher in Asia compared to developed countries [2, 4]. The oral cancer prevalence in Iran and other countries in South Asia such as India, Pakistan and Bangladesh is 20-36.3 per 100,000 people [2].
Oral Squamous Cell Carcinoma (OSCC) is the most common malignancy of oral cavity and accounts for at least 90% of all oral malignancies [4, 5]. Currently, the OSCC is diagnosed based on oral examination and histopathological findings [1, 6]. Biopsy of suspected malignancies is the standard method to determine their nature [6, 7]. In addition to the ailment caused by invasive biopsy, it is possible that precancerous non-homogenous lesions prevent determining the site of the biopsy which is significant in the histopathological assessment of oral cancer. Grading is the histopathological assessment of lesions’ degree of similarity to normal squamous epithelium and a method for evaluating the amount of creatine production. Lesions are graded on a three-point (grade I, II, III) or four-point scales. Poorly differentiated lesions have higher grades [8]. Although there are significant advances in treatment of oral cancer including surgery, radiation therapy, chemotherapy, or their combination, but about 50% of patients suffering from oral cancer survive for 5 years [9, 10]. There is still no effective method to accurately and easily screen oral cancer.
Diagnosis of novel biomarkers is essential for the detection of early malignant oral lesions, especially in those at higher risk [11]. Saliva is a good candidate for detecting biomarkers of oral cancer. Saliva is produced by three main salivary glands including parotid glands, submandibular glands, and sublingual glands, and to a lesser extent by the sub-salivary glands in the lips, cheeks, tongue, palate, and glandular ducts [12]. Using saliva to screen oral cancer is an easier and less invasive method compared to blood sampling or biopsy. Moreover, it is more easily tolerated by patients [6].
Zinc finger protein 510 is a protein that is encoded by the ZNF510 gene in humans. ZNF510 protein is a member of a zinc-finger protein family with about 30 amino acids and zinc ions. It is involved in the control of cell growth, proliferation, differentiation, and programmed death. This protein has been observed in the saliva of patients with OSCC [9]. To our knowledge, only one study has assessed the level of ZNF510 in the saliva of patients with OSCC [9, 13]. In this regard, this study aims to compare the level of ZNF510, as a novel biomarker for diagnosis of OSCC in the early stages, in the saliva of patients with OSCC and healthy individuals.
Materials and Methods
This cross-sectional analytical study was conducted on patients with OSCC and healthy individuals referred to Omid and Imam Reza hospitals affiliated to Mashhad University of Medical Sciences in Iran in 2019. In patients with OSCC, clinical and pathological examination had confirmed the presence of disease and had other inclusion criteria; i.e. age >18 years, consent to participate in the study [9]. Exclusion criteria were any systemic autoimmune disease, existence of oral mucosal lesions other than OSCC, and pregnancy or lactation [9]. According to the results of Jou et al. [9] and at the confidence level of 95%, a test power of 80%, and a type I error level of 50%, the sample size was calculated 9 for each group. This number was increased to 21 for further confidence (21 patients with OSCC and 21 healthy individuals). Prior to study, the procedure was explained to participants, and they signed a written informed consent form to enter the study. Then, their demographic characteristics and the lesion characteristics in the patient group (including lesion type, lesion site, and the histopathology grade of lesion) were recorded. Saliva sampling was then performed. Unstimulated saliva was used for saliva sampling because stimulated saliva has a low concentration of biomarkers and makes it difficult to detect [14]. Participants were advised to avoid smoking and alcohol use for 24 hours before collecting saliva. On the day of sampling, patients were not allowed to smoke, eat, drink or brush for 1-1.5 hour before collecting samples. Spitting method was used to collect unstimulated saliva [15]. Participants were asked to collect saliva in their mouths and then put it into a sterile plastic falcon tube. This was done every 60 seconds for 5-15 minutes between 9-12 a.m. Approximately 5 mL of saliva was collected by this method. During sampling, participants were in a sitting position with slightly forward posture, feeling completely comfortable.
The samples kept in the ice flask were transferred to the central laboratory of Mashhad University of Medical Sciences and stored in a refrigerator at -20°C for up to 3 days. The samples were thawed at room temperature and were then centrifuged for 15 minutes (rpm at 3000) immediately after defrosting to separate squamous cells, debris and mucus. The supernatant solution was collected and poured into 1.5-mL microtubes and stored at -80°C in the refrigerator. In the next step, ZNF510 (part per million or PPM) values were evaluated using an ELISA Kit (ZellBio GmbH, Germany) and ELISA method. For this purpose, each sample was examined twice by an experienced technician who was unaware of allocation.
In summary, the expriment steps were as following: Preparation of samples and standardization, adding 40 µl of samples, 10 µl of ZNF510, 50 µl of standard solution, and 50 µl of Streptavidin-HRP and reacting for 60 minutes at 37°C, Washing the plate 5 times with 300 µl diluted buffer, adding 50 µl of chromogen solution A and 50 µl of solution B and incubating for 10 minutes at 37°C, adding 50 µl of stop solution, reading optical density within 10 minutes at a wavelength of 450 nm, performing calculations and presenting reports.
Collected data were analyzed in SPSS software, vertion .23. For this purpose, Mean±SD error of ZNF510 concentration were reported for saliva samples of healthy individuals and patients with OSCC. Kolmogorov-Smirnov, independent t-test, chi-square, Pearson correlation test, Kruskal-Wallis and analysis of covariance were used to analyze the data. Significance level was set at 0.05 in all statistical tests.
Results
In this study, 42 individuals participated including 29 women (69%) and 13 men (31%) with a mean age of 60.62±10.77 years ranged 42-75 years. There was no significant difference between the two groups in terms of gender (P=0.317), but the mean of age in the healthy group was significantly lower than in the patient group (P<0.001).
Table 1 and Figure 1, shows the mean and standard deviation of ZNF510 level. As can be seen, the mean of ZNF510 (ppm) in the patient group was significantly higher than in the healthy group according to the results of independent t-test (P=0.019); however, after considering age as a confounding variable, although the mean of ZNF510 in the patient group was higher, this difference was not significant (P=0.090).


Therefore, age affected the amount of ZNF510. As it can be seen from Figure 2, in both groups, age had a direct but weak and insignificant relationship with ZNF510. In the patient group, the mean of ZNF510 was lower in women than in men, but the difference was not significant (P=0.555). In the healthy group, the mean of ZNF510 was higher in women than in men, but the difference was not significant (P=0.606).

The clinical examination of lesions showed that the lowest and highest mean of ZNF510 were related to ulcer and prominent lesions, respectively. The mean of ZNF510 was not significantly different in terms of lesion type (P=0.522). Furthermore, in terms of lesion site, the lowest and highest mean of ZNF510 were observed on the gums and lips, respectively. The mean of ZNF510 was not significantly different in terms of of lesion site (P=0.089).
Table 2 presents the mean, standard deviation, minimum, maximum, and mean of the ZNF510 level categorized by different grades of lesion histopathology in the patient group. In patients with histopathological grade I, the lowest and highest levels of ZNF510 were reported 12.5 and 83.75 ppm; and in patients with histopathology grade II, they were 107.7 and 167.5 ppm, respectively. The mean of ZNF510 in patients with lesion grade II was significantly higher than in those with lesion grade I (P<0.001). It should be noted that 6 patients had an unknown degree of lesion histopathology. Therefore, the information in Table 2 is related to 15 patients with OSCC.

Discussion
The current study aimed to measure the salivary ZNF510 protein concentration in patients with OSCC compared to healthy individuals. In patients with OSCC, the mean of ZNF510 in those with lesion grade II was significantly higher than in those with lesion grade I. Moreover, the concentration of ZNF510 protein with the prominent exophytic tupe was higher than other clinical features, and based on the lesion site, the mean of ZNF510 in patients with lip lesions was higher than in others. However, the mean of ZNF510 was not significantly different between the two groups in terms of lesion type and site.
As mentioned before, oral cancer is the sixth most prevalent cancer worldwide, accounting for approximately 4% of all cancer cases [16]. Although the oral cavity may be examined directly, oral cancer sometimes is not diagnosed until the final stages. Biopsy and histopathological assessment are the gold standard methods for definitive diagnosis of oral cancer, including OSCC [6]. Studies have shown that saliva can be used for early diagnosis of OSCC along with biomarkers such as metallopeptidase-9 and Chemerin [12]. ZNF proteins are a group of proteins with a wide range of molecular functions involved in the regulation of several cellular processes, and their function is very different. The function of ZNF proteins seems to be related to the control of growth, proliferation, differentiation, and programmed death of cell (apoptosis) [17, 18]. Some studies have examined different types of ZNF proteins in head and neck cancers and OSCC [19-22]. One study by Jou et al. in 2011 examined the amount of ZNF510 protein in the saliva of patients with OSCC [9]. According to them, ZNF510 peptide levels were significantly increased in the saliva of patients with OSCC [9]. They indicated that ZNF510 level was significantly related to the oral cancer stages where it was higher in T3+ T4 stages compared to T1+ T2 stages. Moreover, they reported that the sensitivity and specificity in the early diagnosis and progression stage of the tumor was 96%. They concluded that the detection of ZNF510 peptide level is related to the progression of OSCC, and it is effective and useful for accurate and early diagnosis [9]. In our study, the mean of ZNF510 protein concentration in the saliva of patients with OSCC was significantly higher than in healthy individuals, but this difference was not significant between the two groups after controlling the age factor as a confounding factor, indicating that age factor affects the salivary ZNF510 concentration. Therefore, saliva protein levels are changed by increasing age, and there is an age-related effect on the secretion of a particular compound in saliva. Although the relationship between ZNF510 concentration and age in our study was weak, it can be claimed that the ZNF510 protein concentration in saliva increases by the increase of age.
Conclusion
The ZNF510 protein concentration is significantly higher in patients with OSCC compared to healthy individuals. However, after considering age as a confounding variable, this difference is not significant. It is recommended to conduct more studies by considering the effect of age factor.
It should be noted that the healthy group was selected from among the patients‘ healthy companions (first degree relatives) in order to homogenize the two groups in terms of heritage. One the other hand, the ZNF510 concentration was significantly higher in patients with lesion grade II compared to those with grade I. Therefore, ZNF510 in saliva may play a role as a possible biomarker for early diagnosis and progression of OSCC. However, more studies are needed with higher sample sizes and examinations of lesions grades III and IV in order to generalize the findings. The results of this study can be regarded as the beginning of further studies on the diagnostic methods of OSCC.
Ethical Considerations
Compliance with ethical guidelines
The study has an ethical approval obtained from the Research Ethics Committee of Mashhad University of Medical Sciences (Code: IR.MUMS.DENTISTRY.REC.1397.118).
Funding
This study was extracted from a dissertation in Dentistry approved by Mashhad Dental School. It was funded by the Deputy for Research of MasMashhad University of Medical Sciences.
Authors' contributions
Study design, data collection, writing, editing & review, and final approval: All authors; Conceptualization: Alla Ghazi.
Conflicts of interest
The authors declare no conflict of interest.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018; 68(6):394-424. [DOI:10.3322/caac.21492] [PMID]
- Maleki D, Ghojazadeh M, Mahmoudi SS, Mahmoudi SM, Pournaghi-Azar F, Torab A, et al. Epidemiology of oral cancer in Iran: A systematic review. Asian Pacific Journal of Cancer Prevention. 2015; 16(13):5427-32. [DOI:10.7314/APJCP.2015.16.13.5427] [PMID]
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncology. 2009; 45(4-5):309-16. [DOI:10.1016/j.oraloncology.2008.06.002] [PMID]
- Nasry WHS, Rodriguez-Lecompte JC, Martin CK. Role of COX-2/PGE2 mediated inflammation in oral squamous cell carcinoma. Cancers. 2018; 10(10):348. [DOI:10.3390/cancers10100348] [PMID] [PMCID]
- Andisheh-Tadbir A, Mehrabani D, Heydari ST. Epidemiology of squamous cell carcinoma of the oral cavity in Iran. Journal of Craniofacial Surgery. 2008; 19(6):1699-702. [DOI:10.1097/SCS.0b013e31818c04cc] [PMID]
- Khurshid Z, Zafar MS, Khan RS, Najeeb S, Slowey PD, Rehman IU. Role of salivary biomarkers in oral cancer detection. Advances in Clinical Chemistry. 2018; 86:23-70. [DOI:10.1016/bs.acc.2018.05.002] [PMID]
- Feitosa SG, Viana KF, Luna ECM, Costa FWG, Cavalcante RB, Chaves FN, et al. Immunohistochemical evaluation of GLUT-3 and GLUT-4 in oral epithelial dysplasia and oral squamous cell carcinoma. Asian Pacific Journal of Cancer Prevention. 2018; 19(7):1779-83. [DOI:10.22034/APJCP.2018.19.7.1779]
- Jou YJ, Lin CD, Lai CH, Tang CH, Huang SH, Tsai MH, et al. Salivary zinc finger protein 510 peptide as a novel biomarker for detection of oral squamous cell carcinoma in early stages. Clinica Chimica Acta. 2011; 412(15-16):1357-65. [DOI:10.1016/j.cca.2011.04.004] [PMID]
- Thomson PJ. Perspectives on oral squamous cell carcinoma prevention-proliferation, position, progression and prediction. Journal of Oral Pathology & Medicine. 2018; 47(9):803-7. [DOI:10.1111/jop.12733] [PMID]
- Wong DT. Salivary diagnostics powered by nanotechnologies, proteomics and genomics. Journal of the American Dental Association. 2006; 137(3):313-21. [DOI:10.14219/jada.archive.2006.0180] [PMID]
- Neville B, Damm DD, Allen C, Bouquot J. Oral and maxillofacial pathology.4th ed. Philadelphia: Elsevier Saunders; 2016. https://books.google.com/books?id=tzc_vgAACAAJ&printsec=frontcover&dq=editions:ISBN1455770523
- Hema Shree K, Ramani P, Sherlin H, Sukumaran G, Jeyaraj G, Don KR, et al. Saliva as a diagnostic tool in oral squamous cell carcinoma: A systematic review with meta analysis. Pathology & Oncology Research. 2019; 25(2):447-53. [DOI:10.1007/s12253-019-00588-2] [PMID]
- Gualtero DF, Suarez Castillo A. Biomarkers in saliva for the detection of oral squamous cell carcinoma and their potential use for early diagnosis: A systematic review. Acta Odontologica Scandinavica. 2016; 74(3):170-7. [DOI:10.3109/00016357.2015.1110249] [PMID]
- Malamud D. Saliva as diagnostic fluid. BMJ. 1992; 305(6847):207-8. [DOI:10.1136/bmj.305.6847.207] [PMID] [PMCID]
- West IC. Radicals and oxidative stress in diabetes. Diabetic Medicine. 2000; 17(3):171-80. [DOI:10.1046/j.1464-5491.2000.00259.x] [PMID]
- Omura K. Current status of oral cancer treatment strategies: Surgical treatments for oral squamous cell carcinoma. International Journal of Clinical Oncology. 2014; 19(3):423-30. [DOI:10.1007/s10147-014-0689-z] [PMID]
- Cassandri M, Smirnov A, Novelli F, Pitolli C, Agostini M, Malewicz M, et al. Zinc-finger proteins in health and disease. Cell Death Discovery. 2017; 3:17071. [DOI:10.1038/cddiscovery.2017.71] [PMID] [PMCID]
- Ladomery M, Dellaire G. Multifunctional zinc finger proteins in development and disease. Annals of Human Genetics. 2002; 66(Pt 5-6):331-42. [DOI:10.1017/S0003480002001215]
- Kurihara-Shimomura M, Sasahira T, Nakamura H, Nakashima C, Kuniyasu H, Kirita T. Zinc finger AN1-type containing 4 is a novel marker for predicting metastasis and poor prognosis in oral squamous cell carcinoma. Journal of Clinical Pathology. 2018; 71(5):436-41. [DOI:10.1136/jclinpath-2017-204770] [PMID]
- Wang H, Deng X, Zhang J, Ou Z, Mai J, Ding S, et al. Elevated expression of zinc finger protein 703 promotes cell proliferation and metastasis through PI3K/AKT/GSK-3β signalling in oral squamous cell carcinoma. Cellular Physiology and Biochemistry. 2017; 44(3):920-34. [DOI:10.1159/000485360] [PMID]
- Ma H, Yang F, Lian M, Wang R, Wang H, Feng L, et al. Dysregulation of zinc finger protein, X-linked (ZFX) impairs cell pro-liferation and induces apoptosis in human oral squamous cell carcinorma. Tumour Biology. 2015; 36(8):6103-12. [DOI:10.1007/s13277-015-3292-7] [PMID] [PMCID]
- Ko CP, Yang LC, Chen CJ, Yeh KT, Lin SH, Yang SF, et al. Expression of myeloid zinc finger 1 and the correlation to clinical aspects of oral squamous cell carcinoma. Tumour Biology. 2015; 36(9):7099-105. [DOI:10.1007/s13277-015-3419-x] [PMID]
Acknowledgements
The authors would like to thank the Vice-Chancellor for Research of Mashhad University of Medical Sciences for the financial support, and all participants for their cooperation.
Type of Study: Original |
Subject:
Basic Medical Science
Received: 2020/10/21 | Accepted: 2021/12/25 | Published: 2021/12/31
Received: 2020/10/21 | Accepted: 2021/12/25 | Published: 2021/12/31
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |


