

Volume 27, Issue 4 (Autumn 2021)
Intern Med Today 2021, 27(4): 434-449 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shokri H, Minooieanhaghigh M, Sharifzadeh A. The Synergistic Activity of Eugenol and Fluconazole on the Induction of Necrosis and Apoptosis in Candida Krusei Isolates of HIV+ Patients With Oral Candidiasis. Intern Med Today 2021; 27 (4) :434-449
URL: http://imtj.gmu.ac.ir/article-1-3650-en.html
URL: http://imtj.gmu.ac.ir/article-1-3650-en.html
1- Department of Pathobiology, Faculty of Veterinary Medicine, Amol University of Special Modern Technologies, Amol, Iran.
2- Department of Microbiology, Faculty of Medicine, Gonabad University of Medical Sciences, Gonabad, Iran. ,drminooeian@gmu.ac.ir
3- Department of Microbiology and Immunology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran.
2- Department of Microbiology, Faculty of Medicine, Gonabad University of Medical Sciences, Gonabad, Iran. ,
3- Department of Microbiology and Immunology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran.
Full-Text [PDF 4973 kb]
(1197 Downloads)
| Abstract (HTML) (2886 Views)
Full-Text: (2252 Views)
1. Introduction
The oral cavity contains more than 200 microbial species, so the incidence of bacterial and fungal infections in the mouth is high. The most common bacterial flora in saliva, tongue, and gums include the Streptococcus species [1]. Candida species are also part of the coexisting fungal flora of the oral cavity, which has different oral transport rates in different age groups and background conditions. These predisposing conditions include immunosuppressive diseases or infections, such as human immunodeficiency virus, diabetes mellitus, long-term corticosteroid therapy, organ transplantation, and chemotherapy for cancer. Under these conditions, a weakened immune system gives the candidate yeast in the body a chance to multiply and grow and cause disease. The highest rates of oral candidiasis are reported in healthy children, the elderly, and AIDS patients [2].
Oral candidiasis is often caused by Candida albicans, Candida glabrata, Candida tropicalis, Candida dublinensis, Candida parapsilosis, and Candida krusei [3]. In recent years, oral colonization with Candida krusei has become common as an emerging yeast agent, especially in patients with AIDS that accounts for about 20% of yeasts isolated from the culture of AIDS patients [4]. Despite the wide range of effective antifungal and antiviral therapies that improve the immune system in AIDS patients, one of the main problems in the treatment of AIDS patients with oral candidiasis is drug resistance due to prolonged treatment period, recurrent relapse, and lack of various antifungal drugs on the market. Multiple studies have shown that Candida krusei, as a common oral Candida species in this group of patients, has shown resistance to some antifungal drugs such as azoles, which has led to treatment failure in treated patients [5].
The use of natural antimicrobial compounds in treating diseases has been common since ancient times. Today, the use of herbal medicines and their pure compounds in treating some infectious diseases has opened new horizons for patients. Extensive research is being done in Iran and the world on natural antifungal compounds [6]. One of these pure compounds is eugenol (4-allyl 2-methoxyphenyl), an allyl derivative of guaiacol. This compound is a member of the phenylpropanoid family with a molecular weight of 164.20 g/mol. It forms a sticky solution in water, while it dissolves well in organic solvents. Cloves, cinnamon, and nutmeg contain significant amounts of this compound [7]. Eugenol is effective in vitro against yeasts, colored and clear filamentous fungi, and mycotoxin-producing fungi also its inhibitory effects on various Candida species have been demonstrated in previous studies [8, 9]. Studies have shown that eugenol has an antifungal effect against Candida yeasts in the range of 350 to 500 μg/mL [10]. Researchers have also demonstrated that combining pure herbal substances with antifungal chemical drugs can significantly help treat patients with candidiasis by reducing the dose of chemical drugs, reducing side effects, and creating synergistic impact [11].
To date, no studies have been performed on the necrotic and apoptotic effects of the combination of eugenol with fluconazole on Candida species. In this study, we evaluated the synergistic effects of antifungal, necrosis, and apoptosis of the natural substance of eugenol in combination with fluconazole against candidate strains isolated from the mouth of AIDS patients. Our results can help meet the scientific needs of the country, commercialization, and manufacture of pharmaceutical products based on natural compounds available in Iran for the treatment of oral candidiasis in humans.
2. Materials and Methods
Fungal isolates and their identification
This study is experimental-laboratory research. In this study, ten clinical strains of Candida krusei (C.k1-10) (isolated from the mouth of HIV+ patients) and one standard strain of Candida krusei (ATCC6258) were used. All yeast isolates were cultured on a Saburaud dextrose agar (Merck) medium and incubated at 37°C for 2 to 3 days. All Candida krusei isolates were identified using standard mycological methods such as chromium agar culture (Paris France Company) and sugar uptake and fermentation tests using RAPIDTM kit (Innovative Diagnostic Systems, USA).
Antifungal compounds and their preparation in different dilutions
In this study, a natural herbal compound called eugenol (Sigma-Aldrich, USA) and a chemical antifungal drug called fluconazole (Sigma-Aldrich, USA) were used. For the experiment, double dilutions of eugenol from 9.76 to 5000 μg/mL and double dilutions of fluconazole from 0.031 to 512 μg/mL were prepared. Isopropanol 70% (Merck) and sterile distilled water were used to prepare dilutions of eugenol and fluconazole, respectively.
Preparation of yeast suspension
To prepare a standard yeast suspension, Candida krusei isolates were first cultured in Saburaud dextrose agar and incubated at 37°C for 24 hours. The inoculum suspension was prepared by harvesting five colonies (about 1 mm in diameter) from the fresh culture in 10 mL of physiological saline (0.85% saline). The suspension was vortexed for 15 seconds, and the cell density was determined by McFarland (0.5) method so that the number of yeast cells was finally adjusted to 0.5 × 103 cells/mL [10].
Antifungal susceptibility testing
The anticandidal activity of the studied compounds was evaluated by serial dilution method in liquid medium (microdilution broth) according to the proposed CLSI method called M27-A2 [12]. In this method, the Minimum Inhibitory Concentration (MIC) was determined using RPMI1640 (Merck) medium containing glutamine and glucose with MOPS buffer with pH 7. Also, 96-plate sterile bottom polystyrene plates were used for the experiment, so that in each well, 100 μL of dilutions of the test compounds and 100 μL of yeast suspension (0.5×103 cells/mL) were added. For each yeast, a negative control well (no-yeast pit) and a positive control well (drug-free pit) were considered. Each experiment was repeated twice separately.
The MIC after 24 hours of microplate incubation at 35°C was determined by ELISA reader. To determine the Minimum Fungicidal Concentration (MFC), 100 μL of the concentration specified as the MIC as well as a few concentrations higher than the MIC, which also appeared to be macroscopically clear, was cultured on Saburaud dextrose agar medium and was examined in an incubator at 35°C after 24 hours. The minimum concentrations that inhibited the growth of Candida species were considered as MFCs. The criterion for sensitivity or resistance to fluconazole has been proposed by CLSI [12]: MIC≥ 8 μg/mL is known as susceptible, MIC≥16-32 μg/mL is known as dose-dependent, and MIC≤64 μg/mL is known as resistant.
Checkerboard Synergy Test
A checker synergy test was performed to determine the fractional inhibitory concentration index (FICI) and Fractional Inhibitory Concentration (FIC) [13]. For this purpose, double serial dilutions of eugenol and fluconazole were prepared. In other words, 50 μL of each dilution of eugenol and fluconazole was added to each plate of 96 cells. Also, 100 μL of yeast suspension of 0.5×103 cells/mL was added to each well and incubated at 35°C for 48 h. The FIC rate was calculated according to the following formula:
FIC drug = “MIC combination drug” / “MIC drug alone”
The synergy of drugs (FICI) was obtained by combining FIC eugenol and FIC fluconazole. The interpretation of the checkboard test is as follows: FICI ≤0.5 (synergistic effect), 1 4 (antagonistic effect).
Evaluation of necrosis and apoptosis by flow cytometry
To determine the percentage of necrotic and apoptotic cells in the population of drug-treated yeasts and compare it with the control population, we stained the yeasts with two dyes of annexin V and Propidium Iodide (PI) using the commercial Annexin-V-FLUOS staining Kit (Roche Diagnostics, Mannheim, Germany). For this purpose, standard Candida krusei isolates (2 × 106 cells/mL) were incubated in broth dextrose containing eugenol and fluconazole for 24 hours at 30°C. Yeast cells were harvested by centrifugation and washed with potassium phosphate buffer (0.1 M). Annexin V / PI test was performed according to the protocol of the staining kit using 5 μg of annexin V and 5 μg of PI at 37°C for 20 minutes. Candidate apoptosis was then analyzed using a C6 flow cytometer (BD Biosciences, San Jose, CA, USA). In this analysis, cells that lost their membranes during programmed death transfer phosphatidylserine from the inner surface to the outer surface of the membrane. Accordingly, annexin V is attached to the phosphatidylserine on the outer surface of the cell and is detected by flow cytometry. Also, the PI attached to the DNA of the fragmented nucleus of dead cells is detected by flow cytometry [14].
Statistical analysis
For statistical analysis, a t-test was used to compare the means of the groups and 1-way ANOVA to find the maximum and minimum effect of the studied materials in all groups. P-values less than 0.05 were considered statistically significant.
3. Results
Antifungal Susceptibility Testing
The MIC and MFC values of eugenol and fluconazole against clinical isolates of Candida krusei are presented in Table 1.

The MIC values of Candida krusei yeasts for eugenol ranged from 312.5 to 1250 μg/mL (geometric mean: 755. 06 μg/mL) and for fluconazole from 8 to 128 μg/mL (geometric mean: 60.1 μg/mL). About 81.8% of the tested Candida krusei strains were resistant to fluconazole. The geometric mean values of MFC eugenol and fluconazole were 1173.66 and 120.2 μg/mL, respectively.
Checkboard Test
The FICI index values for eugenol and fluconazole against different strains of Candida krusei are shown in Table 2.
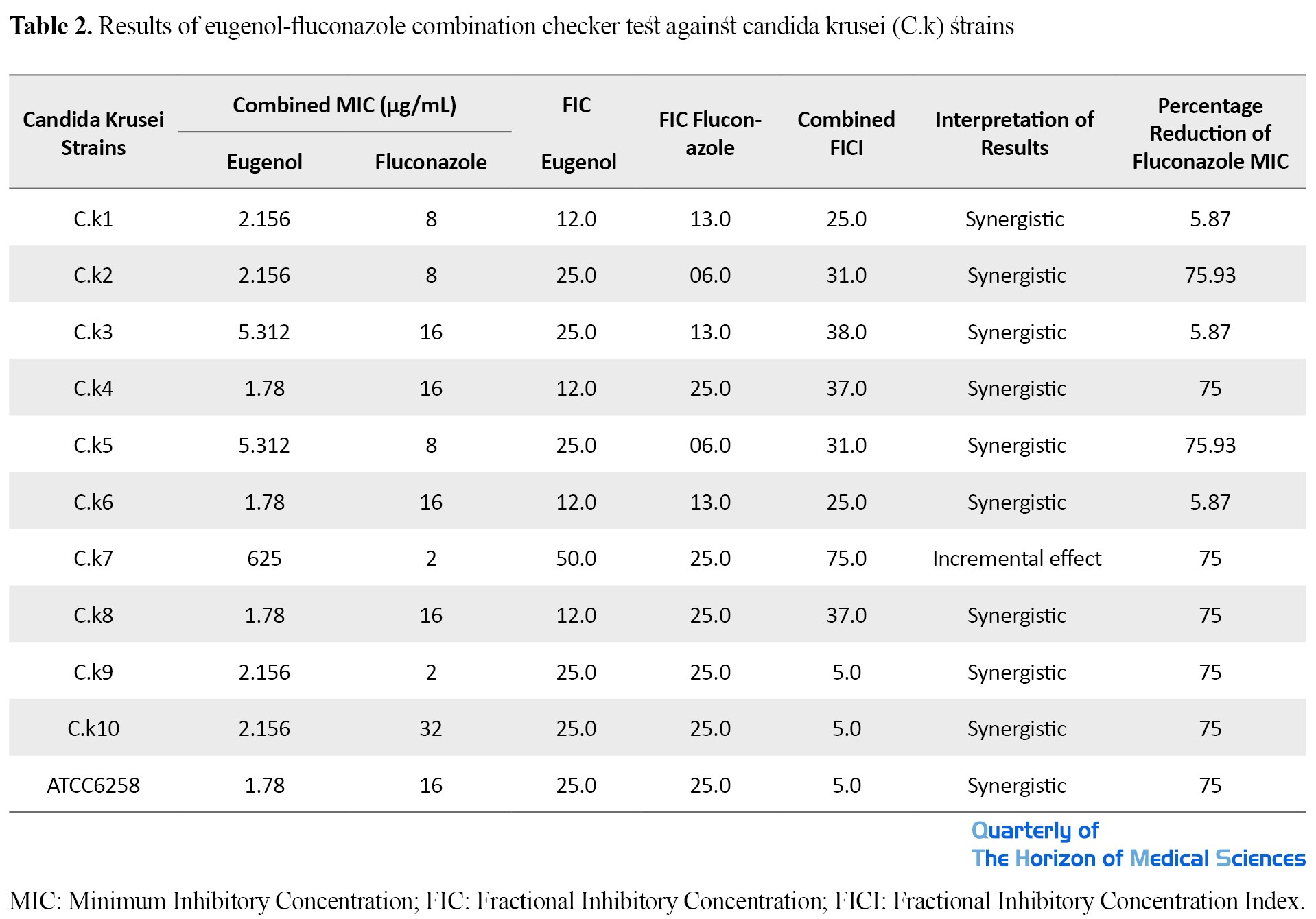
The FICI index range for eugenol + fluconazole was calculated between 0.25 and 0.75 for yeasts. The combination of eugenol with fluconazole had a synergistic effect on 90.9% of clinical isolates of Candida krusei. Statistical analysis of the checkboard test showed a significant synergistic effect between eugenol and fluconazole against the candidate isolates under study (P <0.05). The results showed that eugenol could reduce fluconazole MIC by 81.81% in inhibiting the growth of Candida krusei. Also, in this study, no antagonistic effect was observed between eugenol and fluconazole.
Flow Cytometry Test results
As seen in histograms (Figure 1):
The oral cavity contains more than 200 microbial species, so the incidence of bacterial and fungal infections in the mouth is high. The most common bacterial flora in saliva, tongue, and gums include the Streptococcus species [1]. Candida species are also part of the coexisting fungal flora of the oral cavity, which has different oral transport rates in different age groups and background conditions. These predisposing conditions include immunosuppressive diseases or infections, such as human immunodeficiency virus, diabetes mellitus, long-term corticosteroid therapy, organ transplantation, and chemotherapy for cancer. Under these conditions, a weakened immune system gives the candidate yeast in the body a chance to multiply and grow and cause disease. The highest rates of oral candidiasis are reported in healthy children, the elderly, and AIDS patients [2].
Oral candidiasis is often caused by Candida albicans, Candida glabrata, Candida tropicalis, Candida dublinensis, Candida parapsilosis, and Candida krusei [3]. In recent years, oral colonization with Candida krusei has become common as an emerging yeast agent, especially in patients with AIDS that accounts for about 20% of yeasts isolated from the culture of AIDS patients [4]. Despite the wide range of effective antifungal and antiviral therapies that improve the immune system in AIDS patients, one of the main problems in the treatment of AIDS patients with oral candidiasis is drug resistance due to prolonged treatment period, recurrent relapse, and lack of various antifungal drugs on the market. Multiple studies have shown that Candida krusei, as a common oral Candida species in this group of patients, has shown resistance to some antifungal drugs such as azoles, which has led to treatment failure in treated patients [5].
The use of natural antimicrobial compounds in treating diseases has been common since ancient times. Today, the use of herbal medicines and their pure compounds in treating some infectious diseases has opened new horizons for patients. Extensive research is being done in Iran and the world on natural antifungal compounds [6]. One of these pure compounds is eugenol (4-allyl 2-methoxyphenyl), an allyl derivative of guaiacol. This compound is a member of the phenylpropanoid family with a molecular weight of 164.20 g/mol. It forms a sticky solution in water, while it dissolves well in organic solvents. Cloves, cinnamon, and nutmeg contain significant amounts of this compound [7]. Eugenol is effective in vitro against yeasts, colored and clear filamentous fungi, and mycotoxin-producing fungi also its inhibitory effects on various Candida species have been demonstrated in previous studies [8, 9]. Studies have shown that eugenol has an antifungal effect against Candida yeasts in the range of 350 to 500 μg/mL [10]. Researchers have also demonstrated that combining pure herbal substances with antifungal chemical drugs can significantly help treat patients with candidiasis by reducing the dose of chemical drugs, reducing side effects, and creating synergistic impact [11].
To date, no studies have been performed on the necrotic and apoptotic effects of the combination of eugenol with fluconazole on Candida species. In this study, we evaluated the synergistic effects of antifungal, necrosis, and apoptosis of the natural substance of eugenol in combination with fluconazole against candidate strains isolated from the mouth of AIDS patients. Our results can help meet the scientific needs of the country, commercialization, and manufacture of pharmaceutical products based on natural compounds available in Iran for the treatment of oral candidiasis in humans.
2. Materials and Methods
Fungal isolates and their identification
This study is experimental-laboratory research. In this study, ten clinical strains of Candida krusei (C.k1-10) (isolated from the mouth of HIV+ patients) and one standard strain of Candida krusei (ATCC6258) were used. All yeast isolates were cultured on a Saburaud dextrose agar (Merck) medium and incubated at 37°C for 2 to 3 days. All Candida krusei isolates were identified using standard mycological methods such as chromium agar culture (Paris France Company) and sugar uptake and fermentation tests using RAPIDTM kit (Innovative Diagnostic Systems, USA).
Antifungal compounds and their preparation in different dilutions
In this study, a natural herbal compound called eugenol (Sigma-Aldrich, USA) and a chemical antifungal drug called fluconazole (Sigma-Aldrich, USA) were used. For the experiment, double dilutions of eugenol from 9.76 to 5000 μg/mL and double dilutions of fluconazole from 0.031 to 512 μg/mL were prepared. Isopropanol 70% (Merck) and sterile distilled water were used to prepare dilutions of eugenol and fluconazole, respectively.
Preparation of yeast suspension
To prepare a standard yeast suspension, Candida krusei isolates were first cultured in Saburaud dextrose agar and incubated at 37°C for 24 hours. The inoculum suspension was prepared by harvesting five colonies (about 1 mm in diameter) from the fresh culture in 10 mL of physiological saline (0.85% saline). The suspension was vortexed for 15 seconds, and the cell density was determined by McFarland (0.5) method so that the number of yeast cells was finally adjusted to 0.5 × 103 cells/mL [10].
Antifungal susceptibility testing
The anticandidal activity of the studied compounds was evaluated by serial dilution method in liquid medium (microdilution broth) according to the proposed CLSI method called M27-A2 [12]. In this method, the Minimum Inhibitory Concentration (MIC) was determined using RPMI1640 (Merck) medium containing glutamine and glucose with MOPS buffer with pH 7. Also, 96-plate sterile bottom polystyrene plates were used for the experiment, so that in each well, 100 μL of dilutions of the test compounds and 100 μL of yeast suspension (0.5×103 cells/mL) were added. For each yeast, a negative control well (no-yeast pit) and a positive control well (drug-free pit) were considered. Each experiment was repeated twice separately.
The MIC after 24 hours of microplate incubation at 35°C was determined by ELISA reader. To determine the Minimum Fungicidal Concentration (MFC), 100 μL of the concentration specified as the MIC as well as a few concentrations higher than the MIC, which also appeared to be macroscopically clear, was cultured on Saburaud dextrose agar medium and was examined in an incubator at 35°C after 24 hours. The minimum concentrations that inhibited the growth of Candida species were considered as MFCs. The criterion for sensitivity or resistance to fluconazole has been proposed by CLSI [12]: MIC≥ 8 μg/mL is known as susceptible, MIC≥16-32 μg/mL is known as dose-dependent, and MIC≤64 μg/mL is known as resistant.
Checkerboard Synergy Test
A checker synergy test was performed to determine the fractional inhibitory concentration index (FICI) and Fractional Inhibitory Concentration (FIC) [13]. For this purpose, double serial dilutions of eugenol and fluconazole were prepared. In other words, 50 μL of each dilution of eugenol and fluconazole was added to each plate of 96 cells. Also, 100 μL of yeast suspension of 0.5×103 cells/mL was added to each well and incubated at 35°C for 48 h. The FIC rate was calculated according to the following formula:
FIC drug = “MIC combination drug” / “MIC drug alone”
The synergy of drugs (FICI) was obtained by combining FIC eugenol and FIC fluconazole. The interpretation of the checkboard test is as follows: FICI ≤0.5 (synergistic effect), 1 4 (antagonistic effect).
Evaluation of necrosis and apoptosis by flow cytometry
To determine the percentage of necrotic and apoptotic cells in the population of drug-treated yeasts and compare it with the control population, we stained the yeasts with two dyes of annexin V and Propidium Iodide (PI) using the commercial Annexin-V-FLUOS staining Kit (Roche Diagnostics, Mannheim, Germany). For this purpose, standard Candida krusei isolates (2 × 106 cells/mL) were incubated in broth dextrose containing eugenol and fluconazole for 24 hours at 30°C. Yeast cells were harvested by centrifugation and washed with potassium phosphate buffer (0.1 M). Annexin V / PI test was performed according to the protocol of the staining kit using 5 μg of annexin V and 5 μg of PI at 37°C for 20 minutes. Candidate apoptosis was then analyzed using a C6 flow cytometer (BD Biosciences, San Jose, CA, USA). In this analysis, cells that lost their membranes during programmed death transfer phosphatidylserine from the inner surface to the outer surface of the membrane. Accordingly, annexin V is attached to the phosphatidylserine on the outer surface of the cell and is detected by flow cytometry. Also, the PI attached to the DNA of the fragmented nucleus of dead cells is detected by flow cytometry [14].
Statistical analysis
For statistical analysis, a t-test was used to compare the means of the groups and 1-way ANOVA to find the maximum and minimum effect of the studied materials in all groups. P-values less than 0.05 were considered statistically significant.
3. Results
Antifungal Susceptibility Testing
The MIC and MFC values of eugenol and fluconazole against clinical isolates of Candida krusei are presented in Table 1.

The MIC values of Candida krusei yeasts for eugenol ranged from 312.5 to 1250 μg/mL (geometric mean: 755. 06 μg/mL) and for fluconazole from 8 to 128 μg/mL (geometric mean: 60.1 μg/mL). About 81.8% of the tested Candida krusei strains were resistant to fluconazole. The geometric mean values of MFC eugenol and fluconazole were 1173.66 and 120.2 μg/mL, respectively.
Checkboard Test
The FICI index values for eugenol and fluconazole against different strains of Candida krusei are shown in Table 2.

The FICI index range for eugenol + fluconazole was calculated between 0.25 and 0.75 for yeasts. The combination of eugenol with fluconazole had a synergistic effect on 90.9% of clinical isolates of Candida krusei. Statistical analysis of the checkboard test showed a significant synergistic effect between eugenol and fluconazole against the candidate isolates under study (P <0.05). The results showed that eugenol could reduce fluconazole MIC by 81.81% in inhibiting the growth of Candida krusei. Also, in this study, no antagonistic effect was observed between eugenol and fluconazole.
Flow Cytometry Test results
As seen in histograms (Figure 1):
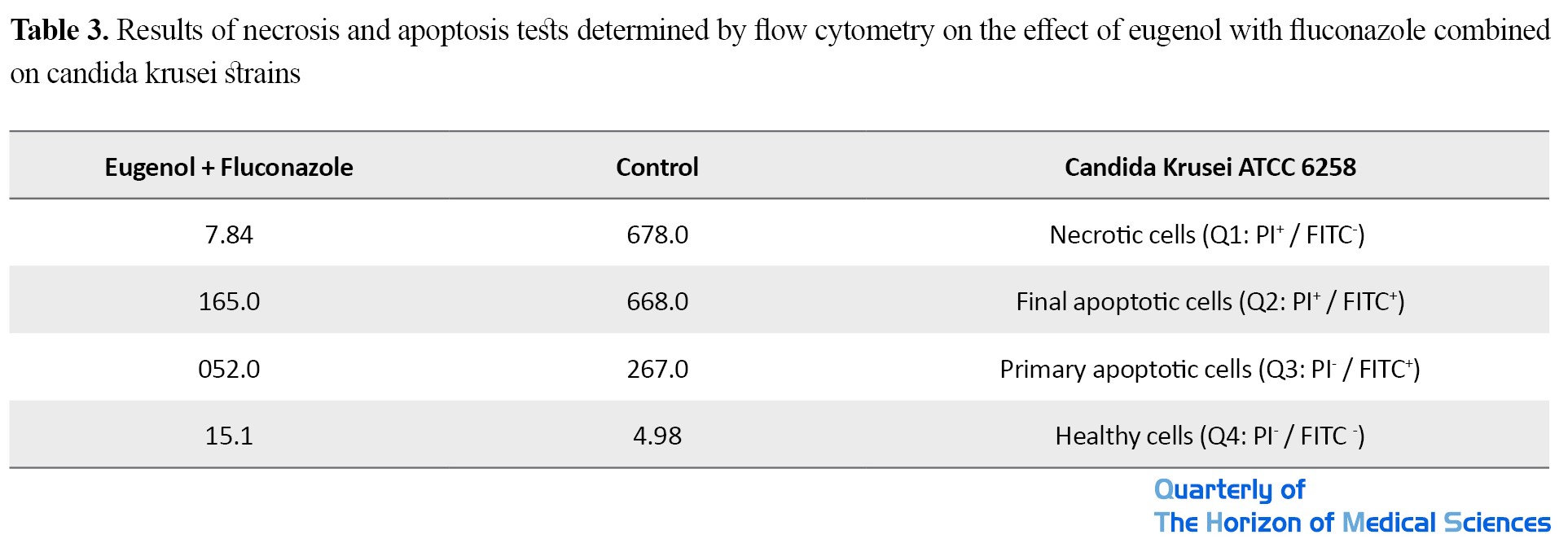
Area Q1: Necrotic cells with PI+ Annexin V-
Area Q2: Cells in the final apoptotic stage with PI+ Annexin V+
Area Q3: Cells in the stage of early apoptosis with PI- Annexin V+
Area Q4: Healthy cells with PI- Annexin V- specificity
Flow cytometry results showed that the combination of eugenol + fluconazole had a significant necrotic effect on clinical isolates of Candida krusei compared to the control group (P <0.05). In contrast, the impact of primary and final apoptosis on this yeast isolate was low (Table 3).
4. Discussion
One of the biggest obstacles to overcoming candida infections of the mouth is using treatment with an antifungal drug, which leads to the emergence of resistance in pathogenic yeasts [15]. Therefore, searching for safe and effective natural ingredients with few side effects is very important. This study aimed to evaluate the synergistic effect of one of the phenylpropanoid compounds in the essential oil of some medicinal plants called eugenol in combination with fluconazole and determine their necrotic and apoptotic effect against clinical isolates of Candida krusei.
In the current study, the mean MIC of fluconazole against Candida krusei isolates was about 60.1 μg/mL and among all the yeasts tested, about 81.8% of them showed significant resistance to fluconazole. The research results by other researchers showed that Candida krusei strains with high MIC values between 50 and 179 μg/mL fluconazole showed high resistance, which is consistent with our findings [13, 16]. Most antifungal drugs used topically and systemically today are azoles. Among azoles, fluconazole is most commonly used to treat candida infections. Although this chemical antifungal drug inhibits fungal cell growth by inhibiting the production of ergosterol in the plasma membrane of Candida species, most strains of Candida krusei are inherently resistant to fluconazole. Therefore, using compounds with high inhibitory activity is necessary to treat infections caused by this species.
The current study results showed that the natural composition of eugenol with a MIC range from 312.5 to 1250 μg/mL has an antifungal effect against resistant Candida krusei isolates. With increasing eugenol concentration in the experimental medium, the growth of candidate isolates gradually decreased. Other researchers have demonstrated the antifungal activity of eugenol against Candida species. In this case, Ahmed et al. [17] showed that eugenol at concentrations of 475-500 μg/mL is an effective natural antifungal compound against candidiasis. In a study by Marcus Arias et al. [9], eugenol concentrations 450-500 μg/mL inhibited the growth of various Candida species, especially Candida krusei. Also, Gallucci et al. [15] reported that eugenol with MIC and MFC values of 880 and 1090 μg/mL, respectively, inhibited the growth and death of Candida krusei strains. Several studies, such as Benis et al. [18] and Braga et al. [19] studies investigated the mechanism of action of eugenol against fungi. Using electron microscopy, the researchers showed that eugenol causes superstructure morphological changes in the membranes of Candida species. In this regard, following the treatment of yeast cells with eugenol at a rate of 500 μg/mL, a significant increase was observed in the number of damaged candidate yeasts with rough and wrinkled surfaces. Researchers have suggested that eugenol, due to its lipophilic properties, can penetrate the fatty acyl chains of the bilayer membrane of yeast plasmalemma and alter the permeability of the cell membrane [20]. Other researchers have shown that eugenol at a concentration of 500 μg/mL inhibits the H +-ATPase activity of Candida species, the ion transport pathway, and the release of H+ stimulated by glucose [21].
Combining molecules with different mechanisms of action is a good strategy for the combined treatment of infectious diseases because it will reduce the side effects, toxicity, and overall dose of the drugs. In our study, the FICI values of the combination of eugenol and fluconazole for Candida krusei isolates were between 0.25 and 0.75. Therefore, a significant decrease (81.81%) in MIC was observed after combining eugenol with fluconazole. Synergistic effects were seen in 90.9% of Candida krusei isolates. Compounds in essential oils are effective in synergistic, augmentative, and antagonistic activities. The synergistic effects of eugenol and antifungal drugs have been evaluated in previous studies. Consistent with our findings, Ahmad et al. [13] showed that eugenol and methyl eugenol combined with fluconazole with FICI values of 0.55-0.31 and 0.58-0.24, respectively, have synergistic effects on Candida species. Other researchers have reported synergistic effects of the combination of eugenol with fluconazole [22], eugenol with amphotericin B [23], and eugenol with nystatin [11]. The role of major constituents of essential oils such as eugenol in such interactions has not been fully studied. Eugenol is thought to interfere with the plasma membrane of the fungal cell and facilitate the penetration of other antifungal drugs such as azoles into the cell.
In this study, flow cytometry showed that the combination of eugenol plus fluconazole has a high necrosis effect and, to a lesser extent, early and late apoptosis on clinical isolates of Candida krusei. According to our extensive research, no studies have been performed on the synergistic effects of necrosis and apoptosis of eugenol with antifungal drugs. Our results are the first laboratory findings reported in the field of combination therapy. However, few reports exist on the necrotic and apoptotic effects of eugenol and its derivatives on Candida species. Lone et al. [24] showed that eugenol derivatives could induce necrosis and apoptosis of yeast cells through the metacaspase pathway. The researchers also showed that eugenol causes yeast necrosis by DNA damage, mitochondrial depolarization, and decreased cytochrome C oxidase activity. Also, in the study of Raja et al. [25], eugenol caused necrosis and apoptosis of yeast cells by reducing ergosterol biosynthesis. In general, the processes of apoptosis and necrosis can be induced by different internal and external stimuli, and their induction, especially in yeast cells, can be considered as a suitable model for monitoring new antifungal agents and treating patients with fungal infections [26].
5. Conclusion
The current study showed that eugenol is a natural monoterpene with antifungal activity and is effective against fluconazole-sensitive oral candida isolates. The combination of eugenol with fluconazole showed a strong synergistic effect that was associated with a significant reduction in the minimum dose of fluconazole. Eugenol in combination with fluconazole also had significant synergistic effects in the development of yeast necrosis. Therefore, combining a natural substance such as eugenol with a chemical drug like fluconazole can both increase the effectiveness of the chemical drug and reduce the dose of the chemical drug, which in turn reduces the side effects of the chemical drug. According to the study results, combination therapy can be a suitable therapeutic approach in cases of treatment failure and resistance to antifungal drugs in AIDS patients with oral candidiasis.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the University Ethics Committee according to the confirmation letter No. 9055/20/98 dated 04/21/2020.
Funding
This research project was approved by the Amol University of New Technologies. An oral sampling of HIV+ patients was referred to Imam Khomeini Hospital of Tehran University of Medical Sciences. Experiments were performed at the Mycology Center of the Amol University of New Technologies in 2020.
Authors' contributions
All authors have contributed to the design, execution, and writing of all sections of the current study.
Conflicts of interest
The authors declared no conflict of interest.
Refrences
Area Q2: Cells in the final apoptotic stage with PI+ Annexin V+
Area Q3: Cells in the stage of early apoptosis with PI- Annexin V+
Area Q4: Healthy cells with PI- Annexin V- specificity
Flow cytometry results showed that the combination of eugenol + fluconazole had a significant necrotic effect on clinical isolates of Candida krusei compared to the control group (P <0.05). In contrast, the impact of primary and final apoptosis on this yeast isolate was low (Table 3).

4. Discussion
One of the biggest obstacles to overcoming candida infections of the mouth is using treatment with an antifungal drug, which leads to the emergence of resistance in pathogenic yeasts [15]. Therefore, searching for safe and effective natural ingredients with few side effects is very important. This study aimed to evaluate the synergistic effect of one of the phenylpropanoid compounds in the essential oil of some medicinal plants called eugenol in combination with fluconazole and determine their necrotic and apoptotic effect against clinical isolates of Candida krusei.
In the current study, the mean MIC of fluconazole against Candida krusei isolates was about 60.1 μg/mL and among all the yeasts tested, about 81.8% of them showed significant resistance to fluconazole. The research results by other researchers showed that Candida krusei strains with high MIC values between 50 and 179 μg/mL fluconazole showed high resistance, which is consistent with our findings [13, 16]. Most antifungal drugs used topically and systemically today are azoles. Among azoles, fluconazole is most commonly used to treat candida infections. Although this chemical antifungal drug inhibits fungal cell growth by inhibiting the production of ergosterol in the plasma membrane of Candida species, most strains of Candida krusei are inherently resistant to fluconazole. Therefore, using compounds with high inhibitory activity is necessary to treat infections caused by this species.
The current study results showed that the natural composition of eugenol with a MIC range from 312.5 to 1250 μg/mL has an antifungal effect against resistant Candida krusei isolates. With increasing eugenol concentration in the experimental medium, the growth of candidate isolates gradually decreased. Other researchers have demonstrated the antifungal activity of eugenol against Candida species. In this case, Ahmed et al. [17] showed that eugenol at concentrations of 475-500 μg/mL is an effective natural antifungal compound against candidiasis. In a study by Marcus Arias et al. [9], eugenol concentrations 450-500 μg/mL inhibited the growth of various Candida species, especially Candida krusei. Also, Gallucci et al. [15] reported that eugenol with MIC and MFC values of 880 and 1090 μg/mL, respectively, inhibited the growth and death of Candida krusei strains. Several studies, such as Benis et al. [18] and Braga et al. [19] studies investigated the mechanism of action of eugenol against fungi. Using electron microscopy, the researchers showed that eugenol causes superstructure morphological changes in the membranes of Candida species. In this regard, following the treatment of yeast cells with eugenol at a rate of 500 μg/mL, a significant increase was observed in the number of damaged candidate yeasts with rough and wrinkled surfaces. Researchers have suggested that eugenol, due to its lipophilic properties, can penetrate the fatty acyl chains of the bilayer membrane of yeast plasmalemma and alter the permeability of the cell membrane [20]. Other researchers have shown that eugenol at a concentration of 500 μg/mL inhibits the H +-ATPase activity of Candida species, the ion transport pathway, and the release of H+ stimulated by glucose [21].
Combining molecules with different mechanisms of action is a good strategy for the combined treatment of infectious diseases because it will reduce the side effects, toxicity, and overall dose of the drugs. In our study, the FICI values of the combination of eugenol and fluconazole for Candida krusei isolates were between 0.25 and 0.75. Therefore, a significant decrease (81.81%) in MIC was observed after combining eugenol with fluconazole. Synergistic effects were seen in 90.9% of Candida krusei isolates. Compounds in essential oils are effective in synergistic, augmentative, and antagonistic activities. The synergistic effects of eugenol and antifungal drugs have been evaluated in previous studies. Consistent with our findings, Ahmad et al. [13] showed that eugenol and methyl eugenol combined with fluconazole with FICI values of 0.55-0.31 and 0.58-0.24, respectively, have synergistic effects on Candida species. Other researchers have reported synergistic effects of the combination of eugenol with fluconazole [22], eugenol with amphotericin B [23], and eugenol with nystatin [11]. The role of major constituents of essential oils such as eugenol in such interactions has not been fully studied. Eugenol is thought to interfere with the plasma membrane of the fungal cell and facilitate the penetration of other antifungal drugs such as azoles into the cell.
In this study, flow cytometry showed that the combination of eugenol plus fluconazole has a high necrosis effect and, to a lesser extent, early and late apoptosis on clinical isolates of Candida krusei. According to our extensive research, no studies have been performed on the synergistic effects of necrosis and apoptosis of eugenol with antifungal drugs. Our results are the first laboratory findings reported in the field of combination therapy. However, few reports exist on the necrotic and apoptotic effects of eugenol and its derivatives on Candida species. Lone et al. [24] showed that eugenol derivatives could induce necrosis and apoptosis of yeast cells through the metacaspase pathway. The researchers also showed that eugenol causes yeast necrosis by DNA damage, mitochondrial depolarization, and decreased cytochrome C oxidase activity. Also, in the study of Raja et al. [25], eugenol caused necrosis and apoptosis of yeast cells by reducing ergosterol biosynthesis. In general, the processes of apoptosis and necrosis can be induced by different internal and external stimuli, and their induction, especially in yeast cells, can be considered as a suitable model for monitoring new antifungal agents and treating patients with fungal infections [26].
5. Conclusion
The current study showed that eugenol is a natural monoterpene with antifungal activity and is effective against fluconazole-sensitive oral candida isolates. The combination of eugenol with fluconazole showed a strong synergistic effect that was associated with a significant reduction in the minimum dose of fluconazole. Eugenol in combination with fluconazole also had significant synergistic effects in the development of yeast necrosis. Therefore, combining a natural substance such as eugenol with a chemical drug like fluconazole can both increase the effectiveness of the chemical drug and reduce the dose of the chemical drug, which in turn reduces the side effects of the chemical drug. According to the study results, combination therapy can be a suitable therapeutic approach in cases of treatment failure and resistance to antifungal drugs in AIDS patients with oral candidiasis.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the University Ethics Committee according to the confirmation letter No. 9055/20/98 dated 04/21/2020.
Funding
This research project was approved by the Amol University of New Technologies. An oral sampling of HIV+ patients was referred to Imam Khomeini Hospital of Tehran University of Medical Sciences. Experiments were performed at the Mycology Center of the Amol University of New Technologies in 2020.
Authors' contributions
All authors have contributed to the design, execution, and writing of all sections of the current study.
Conflicts of interest
The authors declared no conflict of interest.
Refrences
- Shokri H. Immunology of fungal infections. Mashhad: Ferdowsi University of Mashhad (FUM Press); 2002. https://press.um.ac.ir/index.php?option=com_k2&view=item&id=939&lang=fa
- Patil S, Majumdar B, Sarode SC, Sarode GS, Awan KH. Oropharyngeal candidosis in HIV-infectedpatients-An update. Frontiers in Microbiology. 2018; 9(10):980. [DOI:10.3389/fmicb.2018.00980]
- Millsop JW, Fazel N. Oral candidiasis. Clinics in Dermatology. 2016; 34(4):487-94. [DOI:10.1016/j.clindermatol.2016.02.022] [PMID]
- Hosain Pour A, Salari S, Ghasemi Nejad Almani P. [Oropharyngeal candidiasis in HIV/AIDS patients and non-HIV subjects in the Southeast of Iran (Persian)]. Current Medical Mycology. 2018; 4(4):1-6. [DOI:10.18502/cmm.4.4.379] [PMID] [PMCID]
- Ksiezopolska E, Gabaldón T. Evolutionary emergence of drug resistance in candida opportunistic pathogens. Genes. 2018; 9(9):461. [DOI:10.3390/genes9090461] [PMID] [PMCID]
- Salehi Surmaghi H. Medicinal plants and phytotherapy. Tehran: Donyaee Taghazie; 2006.
- Shokri H. Natural compounds as novel antifungal agents. Amol: Amol University of Special Modern Technologies Press, 2018.
- Abbaszadeh S, Sharifzadeh A, Shokri H, Khosravi AR, Abbaszadeh A. [Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi (Persian)]. Journal de Mycologie Médicale. 2014; 24(2):e51-6. [DOI:10.1016/j.mycmed.2014.01.063] [PMID]
- Marcos-Arias C, Eraso E, Madariaga L, Quindós G. In vitro activities of natural products against oral candida isolates from denture wearers. BMC Complementary and Alternative Medicine. 2011; 11:119. [DOI:10.1186/1472-6882-11-119] [PMID] [PMCID]
- Sharifzadeh A, Shokri H. [In vitro synergy of eugenol on the antifungal effects of voriconazole against candida tropicalis and candida krusei strains isolated from the genital tract of mares (Persian)]. Equine Veterinary Journal. 2021; 53(1):94-101. [DOIi:10.1111/evj.13268] [PMID]
- Da Silva ICG, Santos HBP, Cavalcanti YW, Nonaka CFW, de Sousa SA, de Castro RD. Antifungal activity of eugenol and its association with nystatin on candida albicans. Pesquisa Brasileira em Odontopediatria e Clinica Integrada. 2017; 17(1):e3235. [DOI:10.4034/PBOCI.2017.171.16]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Guideline second Edition. CLSI document M27-A3, Clinical and Laboratory Standards Institute, Wayne PA, 2008.
- Ahmad A, Khan A, Khan LA, Manzoor N. In vitro synergy of eugenol and methyleugenol with fluconazole against clinical candida isolates. Journal of Medical Microbiology. 2010; 59(Pt 10):1178-84. [DOI:10.1099/jmm.0.020693-0] [PMID]
- Kolahi M, Tabandeh M, Saremy S, Hosseini S, Hashemitabar M. [The study of apoptotic effect of p-coumaric acid on breast cancer cells MCF-7 (Persian)]. Journal of Shahid Sadoughi University of Medical Sciences. 2016; 24 (3):211-21. https://www.sid.ir/en/journal/ViewPaper.aspx?id=514049
- Gallucci MN, Carezzano ME, Oliva MM, Demo MS, Pizzolitto RP, Zunino MP, et al. In vitro activity of natural phenolic compounds against fluconazole-resistant candida species: A quantitative structure-activity relationship analysis. Journal of Applied Microbiology. 2014; 116(4):795-804. [DOI:10.1111/jam.12432] [PMID]
- Sharifzadeh A, Khosravi AR, Shokri H, Shirzadi H.[ Potential effect of 2-isopropyl-5-methylphenol (thymol) alone and in combination with fluconazole against clinical isolates of candida albicans, C. glabrata and C. krusei (Persian)]. Journal de Mycologie Medicale. 2018; 28(2):294-9. [DOI:10.1016/j.mycmed.2018.04.002] [PMID]
- Ahmad A, Wani MY, Khan A, Manzoor N, Molepo J. Synergistic Interactions of eugenol-tosylate and Its congeners with fluconazole against candida albicans. Plos One. 2015; 10(12):e0145053. [DOI:10.1371/journal.pone.0145053] [PMID] [PMCID]
- Bennis S, Chami F, Chami N, Bouchikhi T, Remmal A. Surface alteration of saccharomyces cerevisiae induced by thymol and eugenol. Letters in Applied Microbiology. 2004; 38(6):454-8. [DOI:10.1111/j.1472-765X.2004.01511.x] [PMID]
- Braga PC, Sasso MD, Culici M, Alfieri M. Eugenol and thymol, alone or in combination, induce morphological alterations in the envelope of candida albicans. Fitoterapia. 2007; 78(6):396-400. [DOI:10.1016/j.fitote.2007.02.022] [PMID]
- Marchese A, Barbieri R, Coppo E, Orhan IE, Daglia M, Nabavi SF, et al. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Critical Reviews in Microbiology. 2017; 43(6):668-89. [DOI:10.1080/1040841X.2017.1295225] [PMID]
- Ahmad A, Khan A, Yousuf S, Khan LA, Manzoor N. Proton translocating ATPase mediated fungicidal activity of eugenol and thymol. Fitoterapia. 2010; 81(8):1157-62. [DOI:10.1016/j.fitote.2010.07.020] [PMID]
- Pemmaraju SC, Pruthi PA, Prasad R, Pruthi V. Candida albicans biofilm inhibition by synergistic action of terpenes and fluconazole. Indian Journal of Experimental Biology. 2013; 51(11):1032-7. [PMID]
- Alves JCO, Ferreira GF, Santos JR, Silva LCN, Rodrigues JFS, Neto WRN, et al. Eugenol induces phenotypic alterations and Increases the oxidative burst in cryptococcus. Frontiers in Microbiology. 2017; 8:2419. [DOI:10.3389/fmicb.2017.02419] [PMID] [PMCID]
- Raja MRC, Srinivasan V, Selvaraj S, Mahapatra SK.Versatile and synergistic potential of eugenol: A review. Pharmaceutica Analytica Acta. 2015; 6(5):367. [DOI:10.4172/2153-2435.1000367]
- Lone SA, Wani MY, Fru P, Ahmad A. Cellular apoptosis and necrosis as therapeutic targets for novel eugenol tosylate congeners against candida albicans. Scientific Reports. 2020; 10(1):1191. [DOI:10.1038/s41598-020-58256-4] [PMID] [PMCID]
- Khan MS, Ahmad I, Cameotra SS. Phenyl aldehyde and propanoids exert multiple sites of action towards cell membrane and cell wall targeting ergosterol in candida albicans. AMB Express. 2013; 3(1):54. [DOI:10.1186/2191-0855-3-54] [PMID] [PMCID]
Type of Study: Original |
Subject:
Basic Medical Science
Received: 2021/01/29 | Accepted: 2021/06/2 | Published: 2021/10/1
Received: 2021/01/29 | Accepted: 2021/06/2 | Published: 2021/10/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






