Volume 28, Issue 3 (Summer 2022)
Intern Med Today 2022, 28(3): 366-381 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Amirsardari B, Saremi A, Malekipouya M. The Effect of an Incremental Training Rehabilitation Period on the Serum Levels of Pentraxin-3 and Osteoprotegerin in Rats With Myocardial Infarction. Intern Med Today 2022; 28 (3) :366-381
URL: http://imtj.gmu.ac.ir/article-1-3871-en.html
URL: http://imtj.gmu.ac.ir/article-1-3871-en.html
1- Department of Physical Education, Faculty of Sports Sciences, Borujard Branch, Islamic Azad University, Borujard, Iran.
2- Department of Sports Physiology, Faculty of Sports Sciences, Arak University, Arak, Iran.
3- Department of Sports Physiology, Faculty of Sports Sciences, Tafarsh Hesabi Professor Branch, Islamic Azad University, Tafarsh, Iran. ,malekipooya@iautb.ac.ir
2- Department of Sports Physiology, Faculty of Sports Sciences, Arak University, Arak, Iran.
3- Department of Sports Physiology, Faculty of Sports Sciences, Tafarsh Hesabi Professor Branch, Islamic Azad University, Tafarsh, Iran. ,
Keywords: Myocardial infarction, Incremental training rehabilitation, Pentraxin-3 (PTX3), Osteoprotegerin
Full-Text [PDF 4772 kb]
(734 Downloads)
| Abstract (HTML) (1560 Views)
Full-Text: (1257 Views)
Introduction
Myocardial infarction (MI) is one of the main causes of death in cardiovascular patients, the first cause of death in the world, and the cause of death of 7 million people in 2016 [1]. It is predicted that this disease will lead to the death of nearly 23.6 million people per year by 2030. In more than 20% of MI patients in the first year after the occurrence, the disease relapses and accounts for the largest increase in life years lost due to disease and death [1]. Therefore, MI with visible inflammation and infiltration of inflammatory cells leads to death if not reduced. Of course, the activation of the inflammatory process immediately after MI is necessary to enter the stage of cell repair and proliferation. With myocardial ischemia, the initial pro-inflammatory response is induced and leads to the destruction of necrotic cell remnants from the MI area [2].
However, inflammation is considered a critical background factor for the initiation of the formation of coronary plaques, their instability, and also their rupture. Also, ongoing inflammatory responses can have adverse effects on left ventricular function and the recovery process after MI [3]. Clinical reports have shown that inflammatory reactions after MI can accelerate the occurrence of atherosclerosis and cause the recurrence of MI [4]. Therefore, it is necessary to control the inflammation caused by MI as soon as possible. Pentroxin-3 (PTX3) and osteoprotegerin (OPG) are among the inflammatory factors in heart patients that were investigated in this study. PTX3 is a recently discovered long-chain protein belonging to the family of pentraxins. This protein is localized in the site of Inflammation and is secreted in different types of skeletal muscle cells, monocytes, macrophages, endothelial cells, smooth muscle walls, and also in atherosclerotic wounds in response to inflammatory stimulation [5].
In previous studies, C-reactive protein (CRP) has been mentioned as the strongest predictor of cardiovascular diseases; but recently, studies have shown that PTX3 was a stronger and more intense predictive factor for cardiovascular patients than C-reactive protein (CRP) [6]. Excessive inflammation in MI patients has a direct relationship with PTX3 protein [7]. OPG protein is an inflammatory marker of 401 amino acids that is related to cardiovascular problems and is associated with an increased risk of atherosclerotic lesions in the general population [8]. OPG and its ligand, receptor activator of nuclear factor (NF-kappaB) ligand (RANKL), is a 380-amino-acid soluble glycoprotein of the tumor necrosis factor (TNF) and TNF-related apoptosis-inducing ligand (TRAIL) [8]. It is a potential new regulatory factor involved in endothelial dysfunction and cardiovascular pathogenesis [9]. It is also involved in inflammatory activities, biomechanical stress, coronary calcification, decreased contractile function, anti-apoptotic activity, congestive heart failure diseases, blood pressure disorders in diabetics, disease Crohn’s artery R, environmental and cerebrovascular diseases, and cardiovascular risk of patients with metabolic syndrome. In addition to medical treatments for MI patients, other different methods exist in complementary medicine to deal with cardiovascular problems. Doing sports exercises is one of the first recommendations for the prevention and rehabilitation of this disease [10]. Physical exercise leads to the improvement of cardiac function and necrotic recovery of the myocardium, and after MI, myocardial oxygenation and ventricular reconstruction prevent subsequent MI events [11].
However, the mechanisms affecting this process, especially after a heart attack, have not been fully clarified. Fukuda et al. showed that 3 to 6 months of endurance training in patients with heart failure and cardiomyopathy led to a significant decrease in PTX3 levels [12]. Also, Basati et al. also reported that aerobic exercise activity reduces the plasma levels of PTX3 in diabetic and non-diabetic coronary patients [13]. In the report of Sponder et al., it was shown that endurance training in cardiac patients did not significantly change OPG glycoprotein [14], which was consistent with the results of Kim et al. [15]. The discussion of inflammatory factors PTX3 and OPG in the immune and inflammatory systems and their answer to increased rehabilitation exercises is completely new. However, in various studies, the positive effects of exercise training on various aspects of heart health in patients with MI have been noted, but the physiological mechanisms with an inflammatory approach are not well understood. Therefore, the present study investigated the effect of an increasing exercise rehabilitation period on the serum levels of PTX3 and OPG in infarcted rats.
Materials and Methods
In this controlled research with post-test, thirty-six 8-week-old male Wistar rats with an average weight of 210±26 g purchased from Pasteur Institute were used. These animals were kept in transparent polycarbonate cages under controlled environmental conditions with a temperature of 22°C±2°C, a humidity of 50±5, and a light-dark cycle of 12:12 h with free access to water and special rat food. After transferring the animals to the research environment for 1 week, they were kept in new conditions and after adapting to the laboratory environment, they were randomly divided into 3 groups of 12 healthy animals (H), myocardial infarction (MI), and myocardial infarction-exercise rehabilitation (MI.EX).
Myocardial infarction (MI) induction protocol
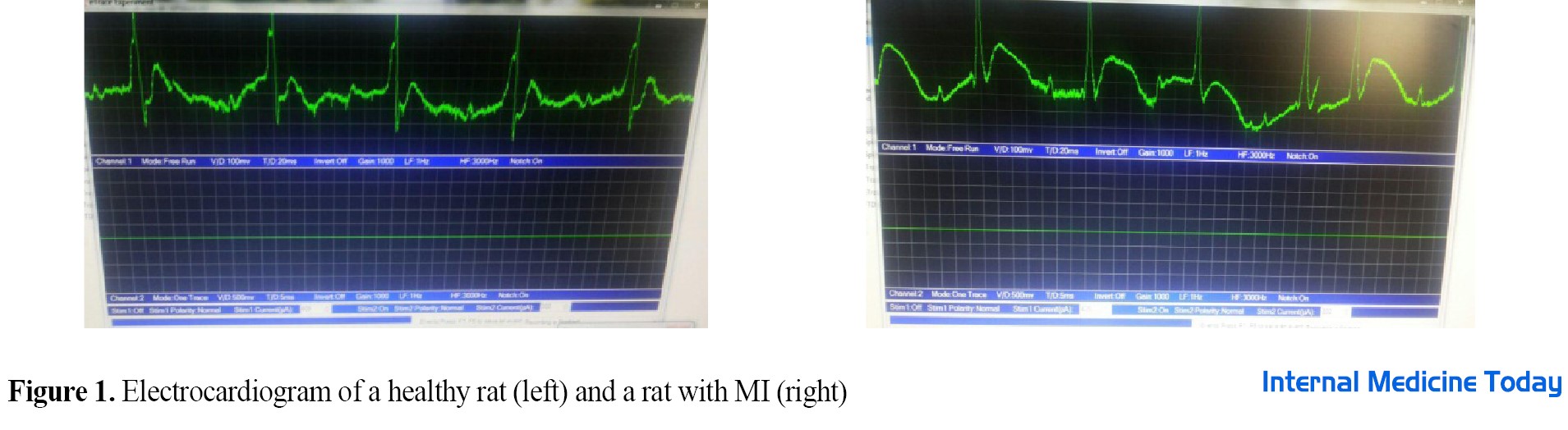
To induce MI, a subcutaneous injection of isoproterenol (ISO, manufactured by Sigma, America) with a dose of 150 mg/kg of body weight was used [16]. Isoproterenol was diluted with normal saline solution (0.05 cc) and administered in two days. It was injected into rats consecutively with an interval of 24 h. This substance is one of the common methods of inducing MI in animal models, especially rats [17]. After 48 h from the last injection, a few rats were randomly selected from each group and subjected to test conditions to ensure the induction of infarction. In this study, cardiac infarction was confirmed based on electrocardiographic changes (ST-segment elevation) along with the increase of cardiac Troponin I (cTnI) enzyme (344.01 pg/mL) (Figure 1). Incremental training rehabilitation protocol
The familiarization phase of rats with the treadmill was performed in the second week for 1 week, 5 days a week, every day for 10 minutes at a speed of 10 m/min [18]. Studies have shown that this amount of training is not enough to significantly change the aerobic capacity of the samples. Rats were conditioned to run by sound and stimulation to avoid approaching, resting, and touching the electric shock section at the end of the device. The incremental aerobic training program, including 8 weeks of running on the treadmill was started on 3 non-consecutive days for 20 minutes at a speed of 12 m/min. In the continuation of the training, 5 minutes were added to the training time every week until it reached 50 minutes (Table 1).

Also, the speed of the treadmill was increased by 1 meter per minute every week until it finally increased to 18 meters per minute. This is a training program with zero slopes performed with 3 minutes of warming up and cooling down at a speed of 7 m/min [19].
Blood sampling and biochemical evaluation
The groups were anesthetized and killed immediately after the end of the training protocol with a combination of ketamine (75 mg/kg) and xylazine (10 mg/kg). At different stages, while observing ethical issues, we tried to avoid any physical abuse and unnecessary methods. After anesthesia, blood sampling was performed directly from the right atrium of the rat heart with 10 cc tube syringes. The collected blood was poured into simple chelated gel tubes and after being placed at room temperature for 10 minutes and clotting, it was centrifuged at 5000 rpm for 5 minutes. Then, the serum samples were placed at -80°C for biochemical analysis. To determine serum PTX3 and OPG values, the ELISA method was used according to the manufacturer’s instructions for East Biopharm China kits (with an intra-assay coefficient of variation less than 10% for both kits and the sensitivity of the measurement method is 0.1 and 0.03 ng/mL, respectively).
Statistical analysis
After confirming the normal distribution of the data using the Shapiro-Wilk test, a one-way analysis of variance (ANOVA) was used to compare the mean between the groups. Statistical analyses were performed using GraphPad statistical software (version 7) at a significant level (P<0.05) and a 95% confidence level.
Results
After the induction of MI and the end of the research, the body weight of the rats was measured in all groups. The results did not show significant changes in the initial weight group after induction and the final weight among the groups (F=3.1 and P=0.057, F=2.3 and P=0.157). Also, the heart weight of rats after the intervention was studied in all groups and the results showed significant changes between H and MI groups (F=4.3 and P=0.04). Also, the ratio of heart to body weight between the MI group and the H and MI.EX groups also had a significant difference (F=5.3 and P=0.047) (Table 2).
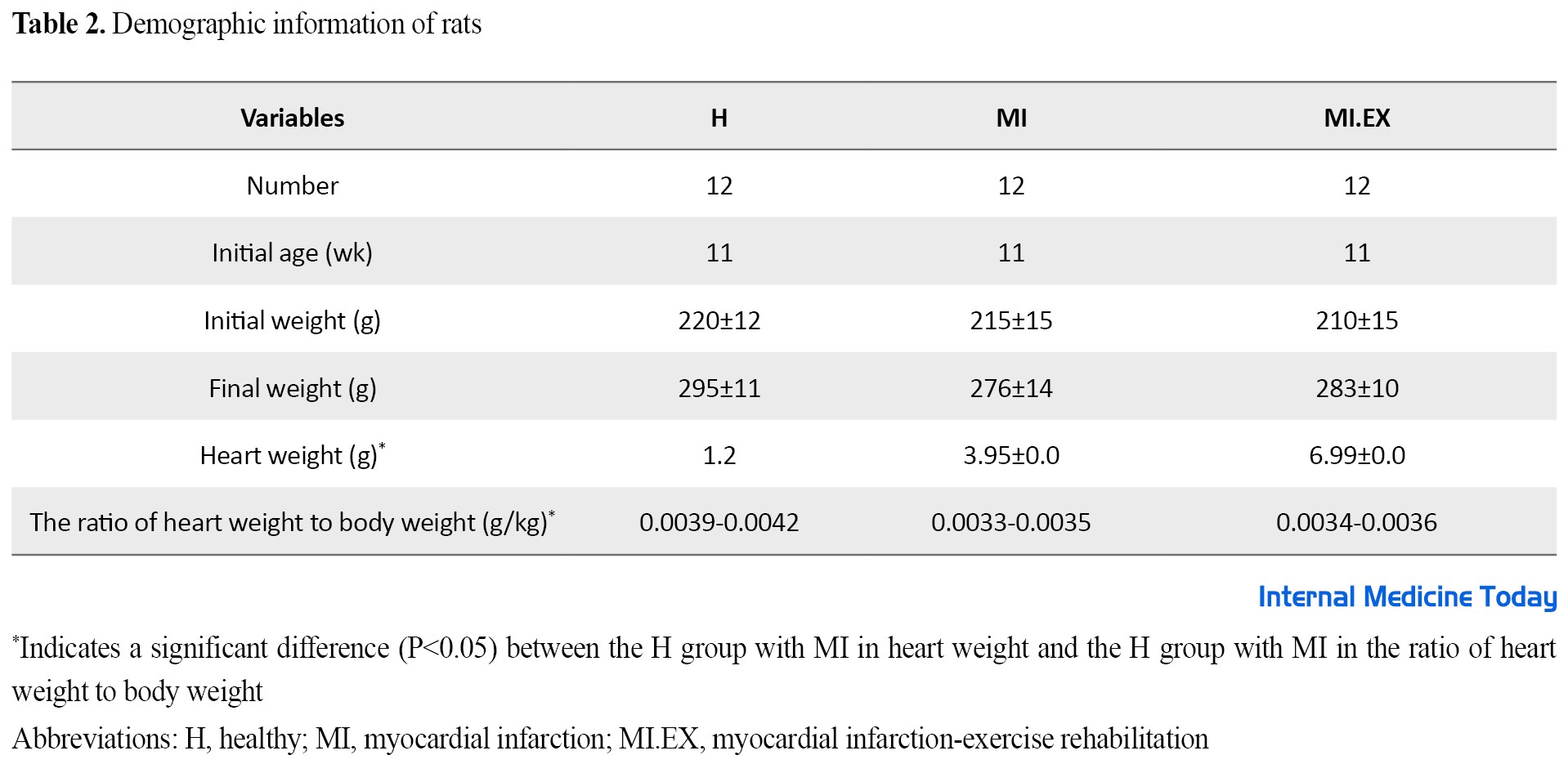
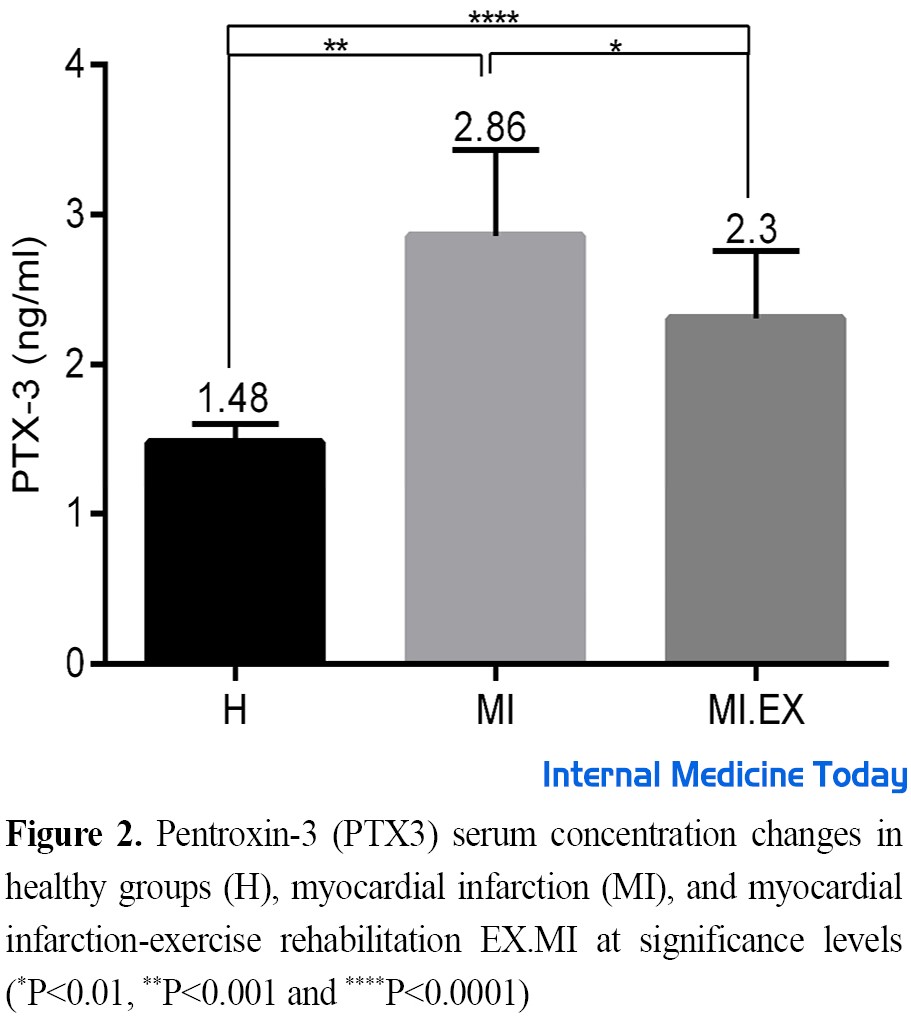
Figure 2 shows the serum concentration of PTX3 in the studied groups after the intervention. The results showed that induction of MI led to a significant increase in the serum levels of PTX3 rats in the intervention groups compared to the healthy group (P=0.0001, F=13). Tukey’s test showed this difference between groups H with MI (F=9, P=0.0001), and H with MI.EX (F=5.5, P=0.002), and MI with MI.EX (F=3.63, P=0.04).
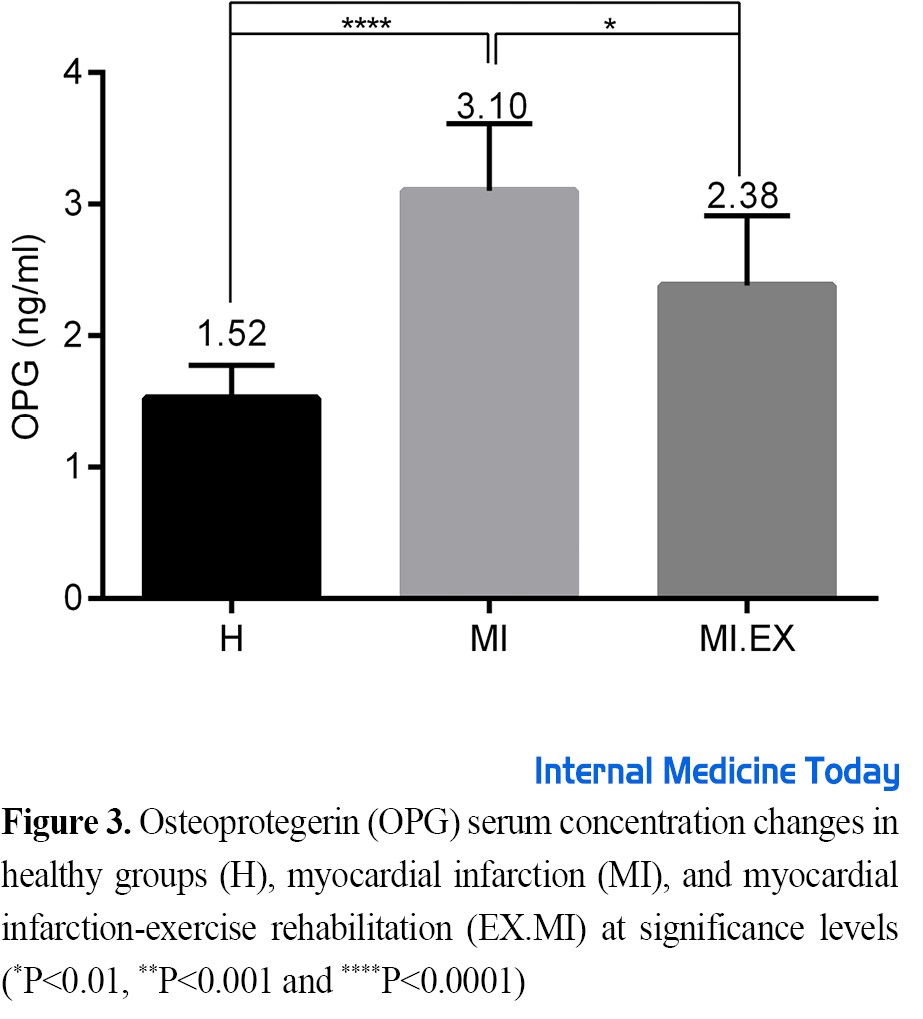
Figure 3 shows the OPG serum concentration in the study groups after the intervention. The results showed that the OPG serum levels of rats increased significantly in the intervention groups compared to the healthy group (P=0.0001, F=11). Tukey’s test showed this difference between groups H with MI (F=10, P=0.0001), and H with MI.EX (F=5.4, P=0.002), and MI with MI.EX (F=4.5, P=0.01).
Discussion
This study was conducted to investigate the changes in serum concentration of PTX3 and OPG in infarcted rats after sports rehabilitation. The research findings showed that the induction of MI caused a significant increase in the serum levels of PTX3 and OPG in the infarct samples. The results of the present study show that eight weeks of incremental endurance rehabilitation training reduced the serum levels of PTX3 and OPG in infarcted samples. Also, the initial and final weight of the groups did not decrease significantly compared to the healthy group. However, heart weight and heart-to-body weight ratio showed a significant decrease in the MI group compared to the healthy group. Cardiac infarction causes abnormal heart function and cardiomyocyte necrosis by causing ischemia. Also, pathological hypertrophy of the left ventricle leads to a decrease in capillary density and an increase in inflammation with the release of molecular proteins related to damage from necrotic cells [12, 20]. Considering that inflammatory responses in the early stages of MI clear infarcted dead cells and matrix remnants and activate reparative processes by myofibroblasts and vascular cells, they may also contribute to scar tissue formation, fibrotic remodeling, cardiac apoptosis, and arrhythmogenic actions. [21]. Prolonged and excessive inflammation plays a prominent role in the pathogenesis of complications and the development of heart failure after infarction. Therefore, interventions to reduce inflammatory symptoms are one of the priorities of cardiac rehabilitation programs. Over the past few years, biomarkers have played a critical role in predicting cardiovascular risks [22]. However, until now, the molecular mechanisms underlying the protective effects and recovery improvement in sports training after MI have not been well clarified. In new studies, PTX3 has been mentioned as one of the strongest predictors of cardiovascular diseases [6]. Also, OPG protein is related to MI and coronary artery patients and increases the risk of heart ulcers in general communities [23]. Arteriosclerosis and MI are associated with increased inflammation-related mortality. Inflammation, mechanical stress, reduced contractile function, and heart failure diseases lead to the expression of PTX3 and OPG genes from atherosclerotic wounds, monocytes, macrophages, and endothelium of heart patients [6, 10]. In studies conducted on MI patients, it has been reported that aerobic exercise prevents negative cardiac inflammatory changes, which confirms the findings of the following research [24]. In the present study, the initial weight of rats did not decrease significantly and the final weights of the groups did not show any significant difference. The reason for the initial weight loss after infarct induction can be seen in the reduction of animal food consumption due to isoproterenol induction [25]. After isoproterenol injection, the weight of the heart of rats significantly decreased compared to the total weight in the MI healthy group, which is consistent with the research results of Amirsardari et al. [26]. Also, increased endurance training led to an increase in heart weight and the ratio of heart weight to total body weight in rats compared to the MI group, which was consistent with other studies [27, 28]. Another finding of the current research showed that the induction of cardiac infarction leads to a significant increase in PTX3 serum levels compared to the healthy group. The results of increased sports rehabilitation after induction of MI showed a significant decrease in PTX3 serum levels. Tunc-Ata et al. showed that a period of exercise training is associated with a significant decrease in PTX3 [29]. In this regard, Fukuda et al. investigated the sports rehabilitation of heart patients and reported that the concentration of PTX3 decreased significantly [12]. In another study, it was reported that aerobic exercise reduces the plasma levels of PTX3 in diabetic and non-diabetic coronary patients [13], which is consistent with the results of the above study. Contrary to the present study, Nakajima et al. investigated the release of PTX3 as an independent inflammatory marker during intense exercise. The results of this study showed that high-intensity exercise increases plasma PTX3 due to exercise [30]. Miyaki et al. also concluded that regular aerobic exercise increases plasma PTX3 concentration by improving HDL, peak oxygen uptake, and arterial dilation in postmenopausal women [7]. The type and intensity of the rehabilitation protocol, health and disease status of the samples, and gender can be the reason for the contradiction. The present study was conducted in infarct samples, with increasing exercise protocol and male rats. In justifying the effectiveness of sports training on PTX3, pentraxins participate in the regulation of inflammatory responses through various reactions. Exercise training stimulates PTX3 interaction with P-selectin [31]. This interaction stops the accumulation of neutrophils in response to the inflammatory stimulus and controls the initial recruitment of neutrophils through a negative feedback mechanism. Therefore, it acts as an intrinsic regulator of the early inflammatory response and probably reduces the severe tissue damage caused by MI inflammation [31]. Also, PTX3 increases the activation of transforming growth factor beta and induces anti-inflammatory responses in macrophages [32]. Since the induction of PTX3 expression through the PI3K/Akt pathway has an atheroprotective effect, positive modulation of PTX3 prevents the activation of inflammatory cascade pathways and protects against cardiovascular wall damage [33]. Therefore, by inducing infarction and increasing PTX3, more anti-inflammatory effects are observed compared to some research [7, 30], and doing sports exercises leads to a decrease in PTX3 and ultimately a decrease in inflammation in heart patients [34]. Another result of the present study was a significant increase in OPG serum levels of rats induced with ISO. OPG biomarker investigation after progressive sports rehabilitation showed a significant decrease compared to the MI group. Regarding OPG protein, Kim et al. showed that combined exercises lead to a significant decrease in OPG protein expression [15]. Consistent with the above results, Sponder et al. also reported that endurance training on people who had at least one of the factors related to heart disease, such as overweight, hypertension, blood pressure, dyslipidemia, diabetes, smoking, and a history of discomfort showed that glycoprotein OPG was associated with a decrease [14]. Also, the research conducted by Davenport et al. showed a decrease in OPG serum levels of overweight and obese people in the sports training program [35]. Contrary to the results of this research, Gaeini et al. showed an increase in OPG gene expression in rats after exercise [36], which was consistent with the results of other studies that led to an increase in OPG following exercise [37]. The cause of the contradiction can be pointed to the healthiness of the research samples, resistance exercises, and the different genders. Cytokine OPG is involved in inflammatory activity, endothelial dysfunction, biomechanical stress, cardiovascular mortality, MI, and coronary artery disease [9].This protein is secreted from different cells such as the heart and blood vessels, arterial wall, vascular smooth muscles, and endothelial cells [38]. In endothelial cells, OPG is secreted from granules due to the stimulation of TNF-α, IL1, and INF-α. In this way, ISO injection leads to an increase in the above markers and ultimately leads to an increase in OPG in heart infarction patients [39]. The increase of inflammatory factors leads to a high regulation of receptor activator of nuclear factor (NF-kappaB) ligand (RANKL) from the vascular muscles, followed by the release of MMP and the destruction of the vessel wall, and finally the creation of plaque, thrombosis, and instability of cardiac function [40]. Reduction of OPG values leads to improvement of clinical symptoms of patients undergoing cardiac sports rehabilitation intervention [41, 42]. It seems that MI disease leaves its effects in the long term, perhaps the length of the research period in the present study is one of the crucial limitations for the detailed examination of the effects of increased exercise training on this disease. Therefore, it is recommended to use a longer period in future studies.
Conclusion
The research findings indicate a decrease in the serum levels of PTX3 and OPG in the exercise group, and it seems that increased exercise rehabilitation can have many clinical benefits by reducing inflammation in heart attack patients.
Ethical Considerations
Compliance with ethical guidelines
In this research, all experimental programs on laboratory animals (familiarization, training, anesthetizing, and killing the animal) were carried out according to the rules of the Wuppertal Ethics Committee on how to treat animals. Also, this research with code IR.IAU.ARAK.REC.1397.007 has been approved by the Research Ethics Committee of Arak University of Medical Sciences.
Funding
The paper was extracted from the PhD. candidate of Babak Amirsardari, Department of Physical Education, Faculty of Sports Sciences, Borujard Branch, Islamic Azad University, Borujard, Iran.
Authors' contributions
All authors contributed equally in preparing all parts of the research.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors are grateful for the cooperation of all the people who participated in this research.
References
Myocardial infarction (MI) is one of the main causes of death in cardiovascular patients, the first cause of death in the world, and the cause of death of 7 million people in 2016 [1]. It is predicted that this disease will lead to the death of nearly 23.6 million people per year by 2030. In more than 20% of MI patients in the first year after the occurrence, the disease relapses and accounts for the largest increase in life years lost due to disease and death [1]. Therefore, MI with visible inflammation and infiltration of inflammatory cells leads to death if not reduced. Of course, the activation of the inflammatory process immediately after MI is necessary to enter the stage of cell repair and proliferation. With myocardial ischemia, the initial pro-inflammatory response is induced and leads to the destruction of necrotic cell remnants from the MI area [2].
However, inflammation is considered a critical background factor for the initiation of the formation of coronary plaques, their instability, and also their rupture. Also, ongoing inflammatory responses can have adverse effects on left ventricular function and the recovery process after MI [3]. Clinical reports have shown that inflammatory reactions after MI can accelerate the occurrence of atherosclerosis and cause the recurrence of MI [4]. Therefore, it is necessary to control the inflammation caused by MI as soon as possible. Pentroxin-3 (PTX3) and osteoprotegerin (OPG) are among the inflammatory factors in heart patients that were investigated in this study. PTX3 is a recently discovered long-chain protein belonging to the family of pentraxins. This protein is localized in the site of Inflammation and is secreted in different types of skeletal muscle cells, monocytes, macrophages, endothelial cells, smooth muscle walls, and also in atherosclerotic wounds in response to inflammatory stimulation [5].
In previous studies, C-reactive protein (CRP) has been mentioned as the strongest predictor of cardiovascular diseases; but recently, studies have shown that PTX3 was a stronger and more intense predictive factor for cardiovascular patients than C-reactive protein (CRP) [6]. Excessive inflammation in MI patients has a direct relationship with PTX3 protein [7]. OPG protein is an inflammatory marker of 401 amino acids that is related to cardiovascular problems and is associated with an increased risk of atherosclerotic lesions in the general population [8]. OPG and its ligand, receptor activator of nuclear factor (NF-kappaB) ligand (RANKL), is a 380-amino-acid soluble glycoprotein of the tumor necrosis factor (TNF) and TNF-related apoptosis-inducing ligand (TRAIL) [8]. It is a potential new regulatory factor involved in endothelial dysfunction and cardiovascular pathogenesis [9]. It is also involved in inflammatory activities, biomechanical stress, coronary calcification, decreased contractile function, anti-apoptotic activity, congestive heart failure diseases, blood pressure disorders in diabetics, disease Crohn’s artery R, environmental and cerebrovascular diseases, and cardiovascular risk of patients with metabolic syndrome. In addition to medical treatments for MI patients, other different methods exist in complementary medicine to deal with cardiovascular problems. Doing sports exercises is one of the first recommendations for the prevention and rehabilitation of this disease [10]. Physical exercise leads to the improvement of cardiac function and necrotic recovery of the myocardium, and after MI, myocardial oxygenation and ventricular reconstruction prevent subsequent MI events [11].
However, the mechanisms affecting this process, especially after a heart attack, have not been fully clarified. Fukuda et al. showed that 3 to 6 months of endurance training in patients with heart failure and cardiomyopathy led to a significant decrease in PTX3 levels [12]. Also, Basati et al. also reported that aerobic exercise activity reduces the plasma levels of PTX3 in diabetic and non-diabetic coronary patients [13]. In the report of Sponder et al., it was shown that endurance training in cardiac patients did not significantly change OPG glycoprotein [14], which was consistent with the results of Kim et al. [15]. The discussion of inflammatory factors PTX3 and OPG in the immune and inflammatory systems and their answer to increased rehabilitation exercises is completely new. However, in various studies, the positive effects of exercise training on various aspects of heart health in patients with MI have been noted, but the physiological mechanisms with an inflammatory approach are not well understood. Therefore, the present study investigated the effect of an increasing exercise rehabilitation period on the serum levels of PTX3 and OPG in infarcted rats.
Materials and Methods
In this controlled research with post-test, thirty-six 8-week-old male Wistar rats with an average weight of 210±26 g purchased from Pasteur Institute were used. These animals were kept in transparent polycarbonate cages under controlled environmental conditions with a temperature of 22°C±2°C, a humidity of 50±5, and a light-dark cycle of 12:12 h with free access to water and special rat food. After transferring the animals to the research environment for 1 week, they were kept in new conditions and after adapting to the laboratory environment, they were randomly divided into 3 groups of 12 healthy animals (H), myocardial infarction (MI), and myocardial infarction-exercise rehabilitation (MI.EX).
Myocardial infarction (MI) induction protocol
To induce MI, a subcutaneous injection of isoproterenol (ISO, manufactured by Sigma, America) with a dose of 150 mg/kg of body weight was used [16]. Isoproterenol was diluted with normal saline solution (0.05 cc) and administered in two days. It was injected into rats consecutively with an interval of 24 h. This substance is one of the common methods of inducing MI in animal models, especially rats [17]. After 48 h from the last injection, a few rats were randomly selected from each group and subjected to test conditions to ensure the induction of infarction. In this study, cardiac infarction was confirmed based on electrocardiographic changes (ST-segment elevation) along with the increase of cardiac Troponin I (cTnI) enzyme (344.01 pg/mL) (Figure 1). Incremental training rehabilitation protocol
The familiarization phase of rats with the treadmill was performed in the second week for 1 week, 5 days a week, every day for 10 minutes at a speed of 10 m/min [18]. Studies have shown that this amount of training is not enough to significantly change the aerobic capacity of the samples. Rats were conditioned to run by sound and stimulation to avoid approaching, resting, and touching the electric shock section at the end of the device. The incremental aerobic training program, including 8 weeks of running on the treadmill was started on 3 non-consecutive days for 20 minutes at a speed of 12 m/min. In the continuation of the training, 5 minutes were added to the training time every week until it reached 50 minutes (Table 1).

Also, the speed of the treadmill was increased by 1 meter per minute every week until it finally increased to 18 meters per minute. This is a training program with zero slopes performed with 3 minutes of warming up and cooling down at a speed of 7 m/min [19].
Blood sampling and biochemical evaluation
The groups were anesthetized and killed immediately after the end of the training protocol with a combination of ketamine (75 mg/kg) and xylazine (10 mg/kg). At different stages, while observing ethical issues, we tried to avoid any physical abuse and unnecessary methods. After anesthesia, blood sampling was performed directly from the right atrium of the rat heart with 10 cc tube syringes. The collected blood was poured into simple chelated gel tubes and after being placed at room temperature for 10 minutes and clotting, it was centrifuged at 5000 rpm for 5 minutes. Then, the serum samples were placed at -80°C for biochemical analysis. To determine serum PTX3 and OPG values, the ELISA method was used according to the manufacturer’s instructions for East Biopharm China kits (with an intra-assay coefficient of variation less than 10% for both kits and the sensitivity of the measurement method is 0.1 and 0.03 ng/mL, respectively).
Statistical analysis
After confirming the normal distribution of the data using the Shapiro-Wilk test, a one-way analysis of variance (ANOVA) was used to compare the mean between the groups. Statistical analyses were performed using GraphPad statistical software (version 7) at a significant level (P<0.05) and a 95% confidence level.
Results
After the induction of MI and the end of the research, the body weight of the rats was measured in all groups. The results did not show significant changes in the initial weight group after induction and the final weight among the groups (F=3.1 and P=0.057, F=2.3 and P=0.157). Also, the heart weight of rats after the intervention was studied in all groups and the results showed significant changes between H and MI groups (F=4.3 and P=0.04). Also, the ratio of heart to body weight between the MI group and the H and MI.EX groups also had a significant difference (F=5.3 and P=0.047) (Table 2).

Figure 2 shows the serum concentration of PTX3 in the studied groups after the intervention. The results showed that induction of MI led to a significant increase in the serum levels of PTX3 rats in the intervention groups compared to the healthy group (P=0.0001, F=13). Tukey’s test showed this difference between groups H with MI (F=9, P=0.0001), and H with MI.EX (F=5.5, P=0.002), and MI with MI.EX (F=3.63, P=0.04).
Figure 3 shows the OPG serum concentration in the study groups after the intervention. The results showed that the OPG serum levels of rats increased significantly in the intervention groups compared to the healthy group (P=0.0001, F=11). Tukey’s test showed this difference between groups H with MI (F=10, P=0.0001), and H with MI.EX (F=5.4, P=0.002), and MI with MI.EX (F=4.5, P=0.01).
Discussion
This study was conducted to investigate the changes in serum concentration of PTX3 and OPG in infarcted rats after sports rehabilitation. The research findings showed that the induction of MI caused a significant increase in the serum levels of PTX3 and OPG in the infarct samples. The results of the present study show that eight weeks of incremental endurance rehabilitation training reduced the serum levels of PTX3 and OPG in infarcted samples. Also, the initial and final weight of the groups did not decrease significantly compared to the healthy group. However, heart weight and heart-to-body weight ratio showed a significant decrease in the MI group compared to the healthy group. Cardiac infarction causes abnormal heart function and cardiomyocyte necrosis by causing ischemia. Also, pathological hypertrophy of the left ventricle leads to a decrease in capillary density and an increase in inflammation with the release of molecular proteins related to damage from necrotic cells [12, 20]. Considering that inflammatory responses in the early stages of MI clear infarcted dead cells and matrix remnants and activate reparative processes by myofibroblasts and vascular cells, they may also contribute to scar tissue formation, fibrotic remodeling, cardiac apoptosis, and arrhythmogenic actions. [21]. Prolonged and excessive inflammation plays a prominent role in the pathogenesis of complications and the development of heart failure after infarction. Therefore, interventions to reduce inflammatory symptoms are one of the priorities of cardiac rehabilitation programs. Over the past few years, biomarkers have played a critical role in predicting cardiovascular risks [22]. However, until now, the molecular mechanisms underlying the protective effects and recovery improvement in sports training after MI have not been well clarified. In new studies, PTX3 has been mentioned as one of the strongest predictors of cardiovascular diseases [6]. Also, OPG protein is related to MI and coronary artery patients and increases the risk of heart ulcers in general communities [23]. Arteriosclerosis and MI are associated with increased inflammation-related mortality. Inflammation, mechanical stress, reduced contractile function, and heart failure diseases lead to the expression of PTX3 and OPG genes from atherosclerotic wounds, monocytes, macrophages, and endothelium of heart patients [6, 10]. In studies conducted on MI patients, it has been reported that aerobic exercise prevents negative cardiac inflammatory changes, which confirms the findings of the following research [24]. In the present study, the initial weight of rats did not decrease significantly and the final weights of the groups did not show any significant difference. The reason for the initial weight loss after infarct induction can be seen in the reduction of animal food consumption due to isoproterenol induction [25]. After isoproterenol injection, the weight of the heart of rats significantly decreased compared to the total weight in the MI healthy group, which is consistent with the research results of Amirsardari et al. [26]. Also, increased endurance training led to an increase in heart weight and the ratio of heart weight to total body weight in rats compared to the MI group, which was consistent with other studies [27, 28]. Another finding of the current research showed that the induction of cardiac infarction leads to a significant increase in PTX3 serum levels compared to the healthy group. The results of increased sports rehabilitation after induction of MI showed a significant decrease in PTX3 serum levels. Tunc-Ata et al. showed that a period of exercise training is associated with a significant decrease in PTX3 [29]. In this regard, Fukuda et al. investigated the sports rehabilitation of heart patients and reported that the concentration of PTX3 decreased significantly [12]. In another study, it was reported that aerobic exercise reduces the plasma levels of PTX3 in diabetic and non-diabetic coronary patients [13], which is consistent with the results of the above study. Contrary to the present study, Nakajima et al. investigated the release of PTX3 as an independent inflammatory marker during intense exercise. The results of this study showed that high-intensity exercise increases plasma PTX3 due to exercise [30]. Miyaki et al. also concluded that regular aerobic exercise increases plasma PTX3 concentration by improving HDL, peak oxygen uptake, and arterial dilation in postmenopausal women [7]. The type and intensity of the rehabilitation protocol, health and disease status of the samples, and gender can be the reason for the contradiction. The present study was conducted in infarct samples, with increasing exercise protocol and male rats. In justifying the effectiveness of sports training on PTX3, pentraxins participate in the regulation of inflammatory responses through various reactions. Exercise training stimulates PTX3 interaction with P-selectin [31]. This interaction stops the accumulation of neutrophils in response to the inflammatory stimulus and controls the initial recruitment of neutrophils through a negative feedback mechanism. Therefore, it acts as an intrinsic regulator of the early inflammatory response and probably reduces the severe tissue damage caused by MI inflammation [31]. Also, PTX3 increases the activation of transforming growth factor beta and induces anti-inflammatory responses in macrophages [32]. Since the induction of PTX3 expression through the PI3K/Akt pathway has an atheroprotective effect, positive modulation of PTX3 prevents the activation of inflammatory cascade pathways and protects against cardiovascular wall damage [33]. Therefore, by inducing infarction and increasing PTX3, more anti-inflammatory effects are observed compared to some research [7, 30], and doing sports exercises leads to a decrease in PTX3 and ultimately a decrease in inflammation in heart patients [34]. Another result of the present study was a significant increase in OPG serum levels of rats induced with ISO. OPG biomarker investigation after progressive sports rehabilitation showed a significant decrease compared to the MI group. Regarding OPG protein, Kim et al. showed that combined exercises lead to a significant decrease in OPG protein expression [15]. Consistent with the above results, Sponder et al. also reported that endurance training on people who had at least one of the factors related to heart disease, such as overweight, hypertension, blood pressure, dyslipidemia, diabetes, smoking, and a history of discomfort showed that glycoprotein OPG was associated with a decrease [14]. Also, the research conducted by Davenport et al. showed a decrease in OPG serum levels of overweight and obese people in the sports training program [35]. Contrary to the results of this research, Gaeini et al. showed an increase in OPG gene expression in rats after exercise [36], which was consistent with the results of other studies that led to an increase in OPG following exercise [37]. The cause of the contradiction can be pointed to the healthiness of the research samples, resistance exercises, and the different genders. Cytokine OPG is involved in inflammatory activity, endothelial dysfunction, biomechanical stress, cardiovascular mortality, MI, and coronary artery disease [9].This protein is secreted from different cells such as the heart and blood vessels, arterial wall, vascular smooth muscles, and endothelial cells [38]. In endothelial cells, OPG is secreted from granules due to the stimulation of TNF-α, IL1, and INF-α. In this way, ISO injection leads to an increase in the above markers and ultimately leads to an increase in OPG in heart infarction patients [39]. The increase of inflammatory factors leads to a high regulation of receptor activator of nuclear factor (NF-kappaB) ligand (RANKL) from the vascular muscles, followed by the release of MMP and the destruction of the vessel wall, and finally the creation of plaque, thrombosis, and instability of cardiac function [40]. Reduction of OPG values leads to improvement of clinical symptoms of patients undergoing cardiac sports rehabilitation intervention [41, 42]. It seems that MI disease leaves its effects in the long term, perhaps the length of the research period in the present study is one of the crucial limitations for the detailed examination of the effects of increased exercise training on this disease. Therefore, it is recommended to use a longer period in future studies.
Conclusion
The research findings indicate a decrease in the serum levels of PTX3 and OPG in the exercise group, and it seems that increased exercise rehabilitation can have many clinical benefits by reducing inflammation in heart attack patients.
Ethical Considerations
Compliance with ethical guidelines
In this research, all experimental programs on laboratory animals (familiarization, training, anesthetizing, and killing the animal) were carried out according to the rules of the Wuppertal Ethics Committee on how to treat animals. Also, this research with code IR.IAU.ARAK.REC.1397.007 has been approved by the Research Ethics Committee of Arak University of Medical Sciences.
Funding
The paper was extracted from the PhD. candidate of Babak Amirsardari, Department of Physical Education, Faculty of Sports Sciences, Borujard Branch, Islamic Azad University, Borujard, Iran.
Authors' contributions
All authors contributed equally in preparing all parts of the research.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors are grateful for the cooperation of all the people who participated in this research.
References
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation. 2020; 141(9):e139-596. [DOI:10.1161/CIR.0000000000000757] [PMCID]
- Zhao ZQ. Velez DA, Wang NP, Hewan-Lowe KO, Nakamura M, Guyton RA, et al. Progressively developed myocardial apoptotic cell death during late phase of reperfusion. Apoptosis. 2001; 6(4):279-90. [DOI:10.1023/A:1011335525219] [PMID]
- Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, Kishore R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circulation Research. 2009; 104(2):e9-18. [DOI:10.1161/CIRCRESAHA.108.188243] [PMID] [PMCID]
- Joshi NV, Toor I, Shah AS, Carruthers K, Vesey AT, Alam SR, et al. Systemic atherosclerotic inflammation following acute myocardial infarction: Myocardial infarction begets myocardial infarction. Journal of the American Heart Association. 2015; 4(9):e001956. [DOI:10.1161/JAHA.115.001956] [PMID] [PMCID]
- Rolph MS, Zimmer S, Bottazzi B, Garlanda C, Mantovani A, Hansson GK. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002; 22(5):e10-4. [DOI:10.1161/01.ATV.0000015595.95497.2F] [PMID]
- Yang HS, Woo JE, Lee SJ, Park SH, Woo JM. Elevated plasma pentraxin 3 levels are associated with development and progression of diabetic retinopathy in Korean patients with type 2 diabetes mellitus. Investigative Ophthalmology & Visual Science. 2014; 55(9):5989-97.[DOI:10.1167/iovs.14-14864] [PMID]
- Miyaki A, Maeda S, Choi Y, Akazawa N, Tanabe Y, Ajisaka R. Habitual aerobic exercise increases plasma pentraxin 3 levels in middle-aged and elderly women. Applied Physiology, Nutrition, and Metabolism. 2012; 37(5):907-11. [DOI:10.1139/h2012-069] [PMID]
- Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circulation Research. 2004; 95(11):1046-57. [DOI:10.1161/01.RES.0000149165.99974.12] [PMID]
- Caidahl K, Ueland T, Aukrust P. Osteoprotegerin: A biomarker with many faces. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010; 30(9):1684-6. [DOI:10.1161/ATVBAHA.110.208843] [PMID]
- Kruzliak P, Berezin A, Kremzer A, Samura T, Benacka R, Mozos I, et al. Global longitudinal strain and strain rate in type two diabetes patients with chronic heart failure: Relevance to osteoprotegerin. Folia Medica. 2016; 58(3):164-73. [DOI:10.1515/folmed-2016-0021] [PMID]
- Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post–myocardial infarction: A systematic review and meta-analysis of randomized controlled trials. American Heart Journal. 2011; 162(4):571-84. [DOI:10.1016/j.ahj.2011.07.017] [PMID]
- Fukuda T, Kurano M, Iida H, Takano H, Tanaka T, Yamamoto Y, et al. Cardiac rehabilitation decreases plasma pentraxin 3 in patients with cardiovascular diseases. European Journal of Preventive Cardiology. 2012; 19(6):1393-400. [DOI:10.1177/1741826711422990] [PMID]
- Basati F, Siahkoohian M, Golabchi A, Moshtaghi M. [Effects of 8 weeks aerobic exercise training on plasma levels of pentraxin3 and C-reactive protein in diabetic and non-diabetic coronary artery disease patients after revascularization interventions (Persian)]. Sport Physiology. 2018; 10(38):163-80. [doi:10.22089/spj.2018.4161.1562]
- Sponder M, Campean IA, Emich M, Fritzer-Szekeres M, Litschauer B, Bergler-Klein J, et al. Endurance training significantly increases serum endocan but not osteoprotegerin levels: a prospective observational study. BMC Cardiovascular Disorders. 2017; 17(1):13.[DOI:10.1186/s12872-016-0452-7] [PMID] [PMCID]
- Kim JY, Kim HJ, Kim CS. Effects of 12-week combined exercise on RANKL/RANK/OPG signaling and bone-resorption cytokines in healthy college females. Journal of Exercise Nutrition & Biochemistry. 2019; 23(1):13-20. [DOI:10.20463/jenb.2019.0003] [PMID] [PMCID]
- Bertinchant JP, Robert E, Polge A, Marty-Double C, Fabbro-Peray P, Poirey S, et al. Comparison of the diagnostic value of cardiac troponin I and T determinations for detecting early myocardial damage and the relationship with histological findings after isoprenaline-induced cardiac injury in rats. Clinica Chimica Acta. 2000; 298(1-2):13-28.[DOI:10.1016/S0009-8981(00)00223-0] [PMID]
- Lobo Filho HG, Ferreira NL, Sousa RB, Carvalho ER, Lobo PL, Lobo Filho JG. Modelo experimental de infarto do miocárdio induzido por isoproterenol em ratos. Brazilian Journal of Cardiovascular Surgery. 2011; 26(3):469-76. [DOI:10.5935/1678-9741.20110024] [PMID]
- MalekiPoya M, Palizvan MR, Saremi A. [The effect of eight weeks of incremental endurance training on the levels of matrix metalloproteinase-1 (MMP1) and thrombosponidine-1 (TSP1) in the rats, induced by myocardial infarction by isoproterenol (Persian)]. Journal of Arak University of Medical Sciences. 2019; 22(3):118-28. [Link]
- Afzalpoura ME, Yousefib MR, Eivaric SH, Ilbeigid S. Changes in blood insulin resistance, GLUT4 & AMPK after continuous and interval aerobic training in normal and diabetic rats. Journal of Applied Pharmaceutical Science. 2016; 6(9):076-81. [DOI:10.7324/JAPS.2016.60911]
- Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: From inflammation to fibrosis. Circulation Research. 2016; 119(1):91-112. [DOI:10.1161/CIRCRESAHA.116.303577] [PMID] [PMCID]
- Newby LK. Inflammation as a treatment target after acute myocardial infarction. The New England Journal of Medicine. 2019; 381(26):2562-3. [DOI:10.1056/NEJMe1914378] [PMID]
- Pokorný J, Staněk V, Vrána M. Sudden cardiac death thirty years ago and at present. The role of autonomic disturbances in acute myocardial infarction revisited. Physiological Research. 2011; 60(5):715-28. [DOI:10.33549/physiolres.932110] [PMID]
- López-Mejías R, Castañeda S, González-Juanatey C, Corrales A, Ferraz-Amaro I, Genre F, et al. Cardiovascular risk assessment in patients with rheumatoid arthritis: The relevance of clinical, genetic and serological markers. Autoimmunity Reviews. 2016; 15(11):1013-30. [DOI:10.1016/j.autrev.2016.07.026] [PMID]
- Ruberti OM, Sousa AS, Viana LR, Pereira Gomes MF, Medeiros A, Gomes Marcondes MC, et al. Aerobic training prevents cardiometabolic changes triggered by myocardial infarction in ovariectomized rats. Journal of Cellular Physiology. 2021; 236(2):1105-15. [DOI:10.1002/jcp.29919] [PMID]
- Upaganlawar A, Gandhi C, Balaraman R. Effect of green tea and vitamin E combination in isoproterenol induced myocardial infarction in rats. Plant Foods for Human Nutrition. 2009; 64(1):75-80. [DOI:10.1007/s11130-008-0105-9] [PMID]
- Amirsardari B, Saremi A, Maleki Pouya M. [Early exercise training attenuates cystatin C and carbohydrate antigen 125 levels after myocardial infarction in rats (Persian)]. Journal of Cell and Tissue. 2021; 12(1). [DOI:10.52547/JCT.12.1.1]
- Ranjbar K, Nazem F, Nazari A, Golami Mr. [Effect of 10 weeks aerobic exercise training on left ventricular systolic function, Caspase-3 level and infarction size in myocardial infarction rat (Persian)]. Journal of Knowledge & Health. 2015; 10(3):16-24. [Link]
- Azamian jazi A, Haffezi MR, Cheraghi J, Abdi H. [The combined effect of endurance training and atorvastatin on the extent of necrosis damageand fibrosis tissue in male wistar rats heart after experimental myocardial infarction (Persian)]. Journal of Ilam University of Medical Sciences. 2016; 23(7):28-38. [Link]
- Tunc-Ata M, Turgut G, Mergen-Dalyanoglu M, Turgut S. Examination of levels pentraxin-3, interleukin-6, and C-reactive protein in rat model acute and chronic exercise. Journal of Exercise Rehabilitation. 2017; 13(3):279-83. [DOI:10.12965/jer.1734920.490] [PMID] [PMCID]
- Nakajima T, Kurano M, Hasegawa T, Takano H, Iida H, Yasuda T, et al. Pentraxin3 and high-sensitive C-reactive protein are independent inflammatory markers released during high-intensity exercise. European Journal of Applied Physiology. 2010; 110(5):905-13. [DOI:10.1007/s00421-010-1572-x] [PMID]
- Bottazzi B, Inforzato A, Messa M, Barbagallo M, Magrini E, Garlanda C, et al. The pentraxins PTX3 and SAP in innate immunity, regulation of inflammation and tissue remodelling. Journal of Hepatology. 2016; 64(6):1416-27.[DOI:10.1016/j.jhep.2016.02.029] [PMID] [PMCID]
- Shiraki A, Kotooka N, Komoda H, Hirase T, Oyama JI, Node K. Pentraxin-3 regulates the inflammatory activity of macrophages. Biochemistry and Biophysics Reports. 2016; 5:290-5. [DOI:10.1016/j.bbrep.2016.01.009] [PMID] [PMCID]
- Fornai F, Carrizzo A, Forte M, Ambrosio M, Damato A, Ferrucci M, et al. The inflammatory protein Pentraxin 3 in cardiovascular disease. Immunity & Ageing. 2016; 13(1):25. [DOI:10.1186/s12979-016-0080-1] [PMID] [PMCID]
- Zempo-Miyaki A, Fujie S, Sato K, Hasegawa N, Sanada K, Maeda S, et al. Elevated pentraxin 3 level at the early stage of exercise training is associated with reduction of arterial stiffness in middle-aged and older adults. Journal of Human Hypertension. 2016; 30(9):521-6. [DOI:10.1038/jhh.2015.105] [PMID]
- Davenport C, Kenny H, Ashley DT, O’Sullivan EP, Smith D, O’Gorman DJ. The effect of exercise on osteoprotegerin and TNF-related apoptosis-inducing ligand in obese patients. European journal of Clinical Investigation. 2012; 42(11):1173-9. [DOI:10.1111/j.1365-2362.2012.02703.x] [PMID]
- Gaeini A, Eslaminejad MB, Choobineh S, Mousavi N, Satarifard S, Shafieineek L. Effects of exercise prior or during pregnancy in high fat diet fed mice alter bone gene expression of female offspring: An experimental study. International Journal of Reproductive BioMedicine. 2017; 15(2):93-100. [PMID]
- Farzanegi P, Niak KE, Habibian M. [The effect of circuit resistance training with Medicago sativa extracts on levels of osteoprotegerin and nuclear factor of Kappa-B in thin girls (Persian)]. Pars Journal of Medical Sciences. 2016; 14(3):27-34. [DOI:10.29252/jmj.14.3.27]
- Fuernau G, Poenisch C, Eitel I, de Waha S, Desch S, Schuler G, et al. Growth-differentiation factor 15 and osteoprotegerin in acute myocardial infarction complicated by cardiogenic shock: A biomarker substudy of the IABP-SHOCK II-trial. European Journal of Heart Failure. 2014; 16(8):880-7. [DOI:10.1002/ejhf.117] [PMID]
- Bernardi S, Bossi F, Toffoli B, Fabris B. Roles and clinical applications of OPG and TRAIL as biomarkers in cardiovascular disease. BioMed Research International. 2016; 2016:1752854. [DOI:10.1155/2016/1752854] [PMID] [PMCID]
- Sandberg WJ, Yndestad A, Øie E, Smith C, Ueland T, Ovchinnikova O, et al. Enhanced T-cell expression of RANK ligand in acute coronary syndrome: Possible role in plaque destabilization. Arteriosclerosis, Thrombosis, and vascular Biology. 2006; 26(4):857-63. [DOI:10.1161/01.ATV.0000204334.48195.6a][PMID] [PMID]
- Calegari L, Nunes RB, Mozzaquattro BB, Rossato DD, Dal Lago P. Exercise training improves the IL-10/TNF-α cytokine balance in the gastrocnemius of rats with heart failure. Brazilian Journal of Physical Therapy. 2018; 22(2):154-60. [DOI:10.1016/j.bjpt.2017.09.004] [PMID] [PMCID]
- Puhl SL, Müller A, Wagner M, Devaux Y, Böhm M, Wagner DR, et al. Exercise attenuates inflammation and limits scar thinning after myocardial infarction in mice. American Journal of Physiology. Heart and Circulatory Physiology. 2015; 309(2):H345-59. [DOI:10.1152/ajpheart.00683.2014] [PMID]
Type of Study: Original |
Subject:
Basic Medical Science
Received: 2022/02/23 | Accepted: 2022/06/22 | Published: 2022/07/1
Received: 2022/02/23 | Accepted: 2022/06/22 | Published: 2022/07/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |